Outsourcing Biologics Fill Finish Manufacturing : Streamlining the Production Process for Biopharmaceutical Companies
Roots Analysis
APRIL 15, 2024
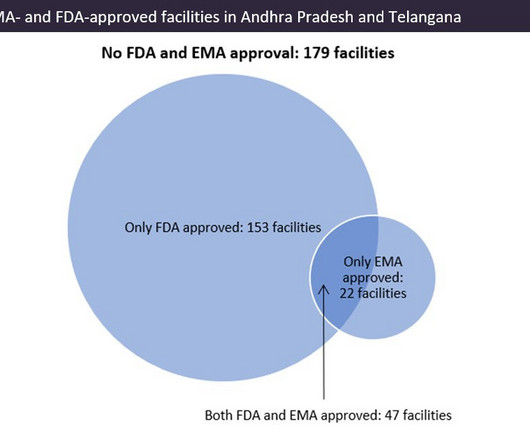
Further, the manufacturing of biologics fill finish is a highly complex and cost-intensive process. For instance, small biotech companies enter into agreements with fill finish manufacturing companies due to lack of in-house capacity.























Let's personalize your content