Bioavailability Enhancement Technologies
Roots Analysis
FEBRUARY 15, 2023
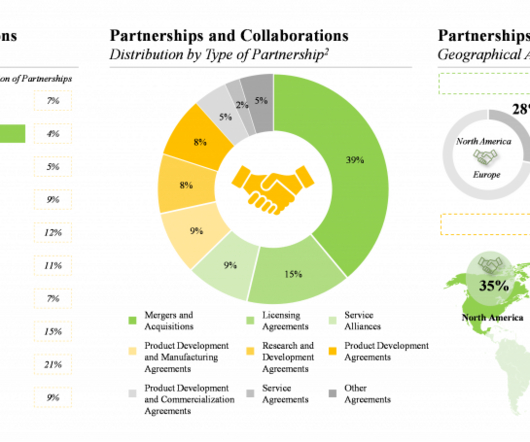
Moreover, it is a well-established fact that the systemic / local absorption and distribution of a therapeutic intervention is directly proportional to its bioavailability. Technology evaluation framework provides a value addition matrix for bioavailability enhancement approaches currently employed by industry stakeholders.




































Let's personalize your content