Considerations for Mobile Health Technology Developers: Part 1
pharmaphorum
SEPTEMBER 29, 2022
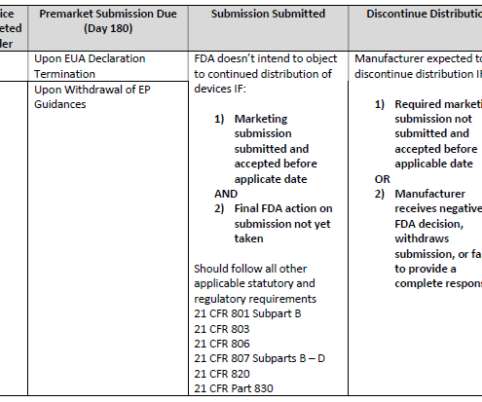
Although mHealth has been gaining in popularity for at least the past decade, before commercializing their mHealth products, developers must determine whether the product is subject to U.S. Food and Drug Administration (FDA) regulation as a medical device. If so, developers must develop and execute on a regulatory strategy.



























Let's personalize your content