Cell Free System market: Current Landscape, Growth Opportunities and Future Market Outlook
Roots Analysis
AUGUST 7, 2023
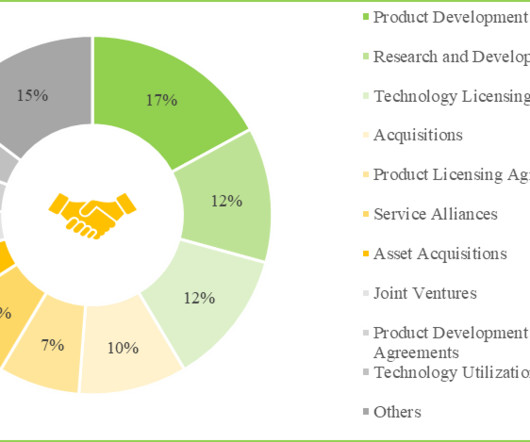
On the other hand, cell free system / cell free expression system are in-vitro platforms that harness the transcription and translation machinery of living cells, in the form of cell lysates, to synthesize various types of biologics, particularly proteins. Interestingly, most of these kits are used for cell-free protein expression.






















Let's personalize your content