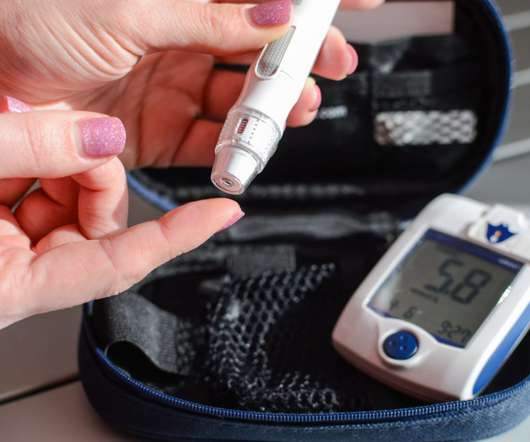
New Type 2 Diabetes Drug Brenzavvy Wins FDA Approval
XTalks
JANUARY 25, 2023
XTALKS WEBINAR: Review of Key Trends for Life Science Commercial Teams in 2023 Register for this free on-demand webinar to learn about the latest trends in sales and marketing campaigns that could help bolster commercial success for life science companies this year.










Let's personalize your content