Pharma benefited from basing business overseas. An international tax effort could spur a rethink.
Bio Pharma Dive
NOVEMBER 28, 2023
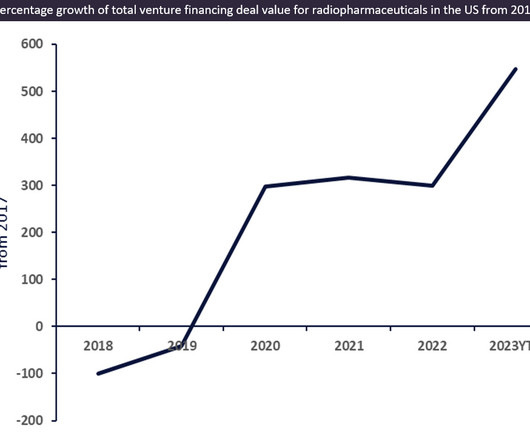
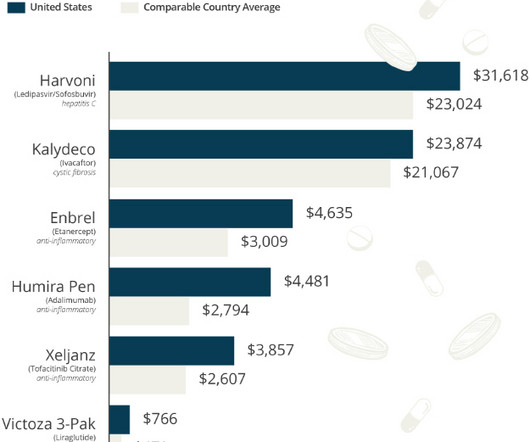
U.S. tax law changes enacted six years ago slashed large pharma companies' rates and saved them billions. Now, a push for an international floor could disrupt their R&D accounting.



































Let's personalize your content