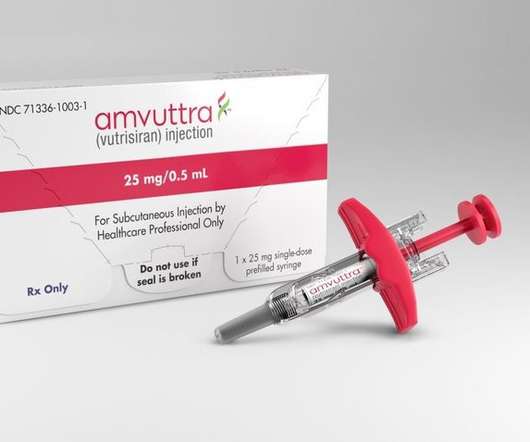
Amvuttra RNAi Therapeutic Wins FDA Approval for Rare Genetic Protein Disorder
XTalks
JUNE 20, 2022
Amvuttra’s FDA approval is based on positive data from the global, randomized, open-label, multicenter HELIOS-A Phase III study from a period of nine months. TTR is made in the liver and is involved in carrying the thyroid hormone thyroxine (T4) and retinol (vitamin A) to the liver.















Let's personalize your content