Itovebi (Inavolisib) Gains FDA Nod for PIK3CA-Mutated HR-Positive, HER2-Negative Breast Cancer
XTalks
OCTOBER 18, 2024
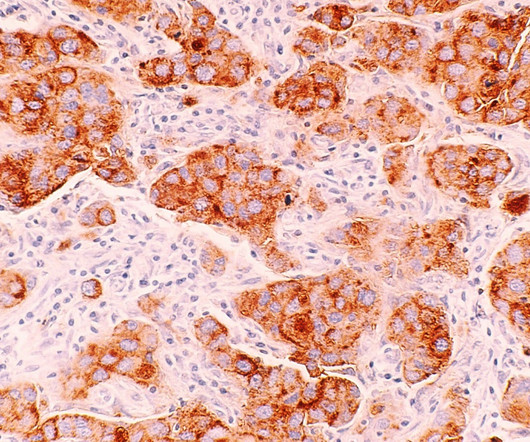
The US Food and Drug Administration (FDA) has approved Itovebi (inavolisib) for the treatment of adults with hormone receptor-positive (HR-positive), human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer with a PIK3CA mutation. Why Target PIK3CA Mutations?








































Let's personalize your content