Adjuvanted Covid-19 vaccine elicits stronger immune response
Drug Discovery World
NOVEMBER 10, 2023
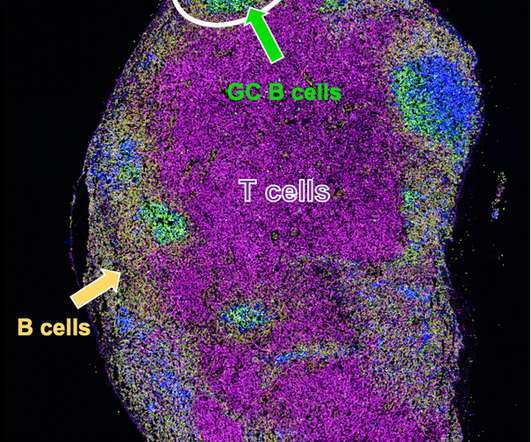
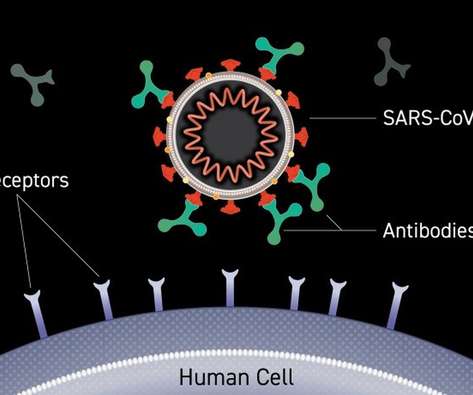
Researchers from Korea conducted a clinical trial to assess the immunogenicity and safety of GBP510/AS03 relative to the ChAdOx1-S vaccine. The participants were split in two groups: those with no prior SARS-CoV-2 infection or Covid-19 vaccination history and those irrespective of these parameters.

































Let's personalize your content