Novartis announces plans to spin off Sandoz business
Pharmaceutical Technology
AUGUST 26, 2022
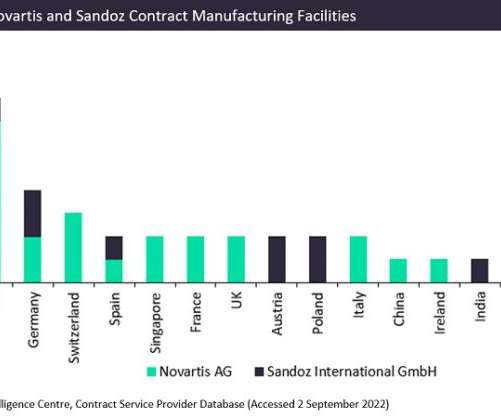
Novartis has announced plans to completely spin off its generics and biosimilars division, Sandoz, to create a new publicly traded standalone firm. The latest development is intended to increase shareholder value by establishing a top European generics firm and a leader in biosimilars internationally.



















Let's personalize your content