First biologic therapy for young children with severe eczema
Drug Discovery World
SEPTEMBER 16, 2022
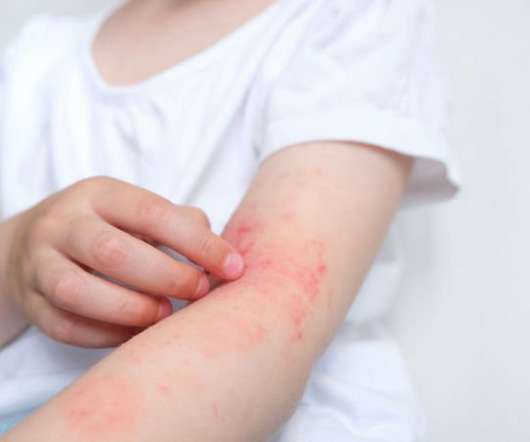
Sponsored by Regeneron and Sanofi, the study of dupilumab in poorly controlled eczema is the first large-scale randomised double-blind trial of a monoclonal antibody for any skin disease in patients aged six months to six years. It’s also incredibly stressful for their families, particularly as children’s sleep is so disturbed. .













Let's personalize your content