Seagen, Genmab win speedy FDA approval for cervical cancer drug
Bio Pharma Dive
SEPTEMBER 21, 2021
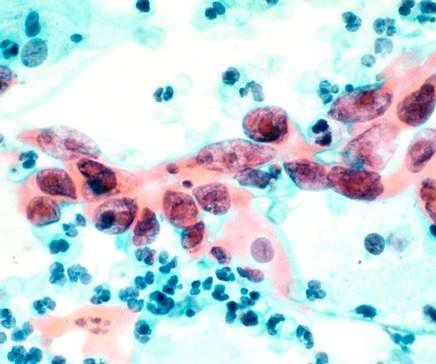
Tivdak, a type of drug known as an antibody-drug conjugate, is the fourth approved medicine for Seagen and its third to reach market since December 2019.































Let's personalize your content