Top biopharmaceutical Covid-19 vaccine companies boosted with over 80% revenue growth
Pharmaceutical Technology
SEPTEMBER 15, 2022
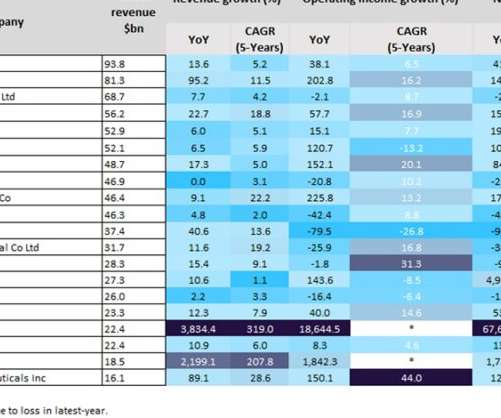
Regeneron’s windfall of $16.1bn in revenue for the year was largely due to its Covid-19 monoclonal antibody therapy, REGEN-COV; due to its ineffectiveness against the Omicron variant, however, its success is expected to be short-lived. Moderna’s Covid-19 vaccine, Spikevax, also contributed to the company’s $18.5bn in revenue for the year.


















Let's personalize your content