Evusheld long-acting antibody combination recommended for approval in the EU for the pre-exposure prophylaxis (prevention) of COVID-19
The Pharma Data
MARCH 25, 2022
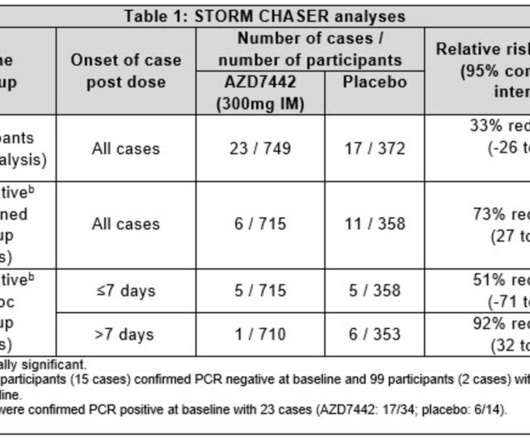
AstraZeneca’s Evusheld (tixagevimab co-packaged with cilgavimab), a long-acting antibody combination, has been recommended for marketing authorisation in the European Union (EU) for the pre-exposure prophylaxis (prevention) of COVID-19 in a broad population of adults and adolescents aged 12 years and older weighing at least 40 kg.





























Let's personalize your content