Evusheld long-acting antibody combination approved in the EU for pre-exposure prophylaxis (prevention) of COVID-19 in a broad population
The Pharma Data
MARCH 29, 2022
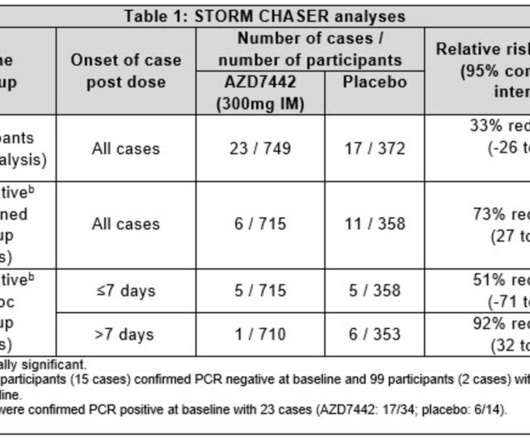
Evusheld significantly reduced the risk of developing symptomatic COVID-19 in PROVENT Phase III trial, with protection lasting at least six months Evusheld retains neutralising activity against the Omicron BA.2 1-3 Evusheld was generally well-tolerated in the trial. Evusheld was generally well-tolerated in the trials.





















Let's personalize your content