ASH23: Pharma branding, Editas’ high bar and clinical trial diversity
Bio Pharma Dive
DECEMBER 11, 2023
Editas had the tall task Monday of convincing ASH attendees its gene therapy for sickle cell disease could improve on Casgevy and Lyfgenia.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Bio Pharma Dive
DECEMBER 11, 2023
Editas had the tall task Monday of convincing ASH attendees its gene therapy for sickle cell disease could improve on Casgevy and Lyfgenia.
Deltaclinical
NOVEMBER 2, 2021
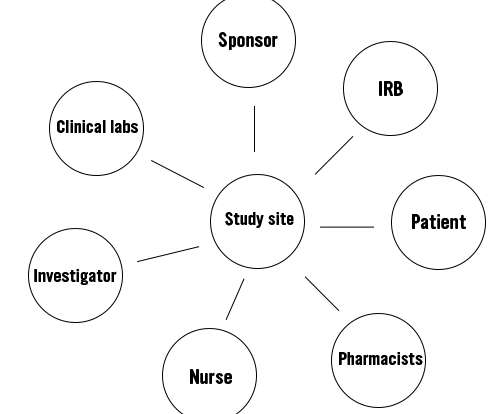
Along with a team of nurses and other stakeholders, the PI will conduct the trial at his/her study site. However, the sponsor will remain the monitor of the trial. The next figure shows the important stakeholders in a clinical trial. Traditionally, clinical trials have never been designed with patient-centricity in mind.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Deltaclinical
NOVEMBER 2, 2021
Along with a team of nurses and other stakeholders, the PI will conduct the trial at his/her study site. However, the sponsor will remain the monitor of the trial. The next figure shows the important stakeholders in a clinical trial. Traditionally, clinical trials have never been designed with patient-centricity in mind.

Deltaclinical
NOVEMBER 2, 2021
Along with a team of nurses and other stakeholders, the PI will conduct the trial at his/her study site. However, the sponsor will remain the monitor of the trial. The next figure shows the important stakeholders in a clinical trial. Traditionally, clinical trials have never been designed with patient-centricity in mind.

Deltaclinical
NOVEMBER 2, 2021
Along with a team of nurses and other stakeholders, the PI will conduct the trial at his/her study site. However, the sponsor will remain the monitor of the trial. The next figure shows the important stakeholders in a clinical trial. Traditionally, clinical trials have never been designed with patient-centricity in mind.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 22, 2022
Drug manufacturers have hailed the Drugs (Eighth Amendment) Rules, 2022 mandating barcode or quick response (QR) code on the label of top 300 brands of formulations from August 1, 2023, saying that QR codes will help identify misbranded or counterfeit medicines as well as recall the products if its quality gets compromised during manufacturing.

WCG Clinical
JUNE 12, 2023
June 12, 2023 — WCG, one of the world’s leading providers of solutions that measurably improve the quality, efficiency, and safety of clinical research, today announces the launch of its new brand identity. In 2022, we touched 90 percent of all clinical trials globally. That’s the beauty of our new brand.”

BioTech 365
MAY 18, 2021
Maxwell Biosciences Announces the CLAROMER™ Brand Biotech Therapeutics Platform as It Advances Toward Clinical Trials Maxwell Biosciences Announces the CLAROMER™ Brand Biotech Therapeutics Platform as It Advances Toward Clinical Trials AUSTIN, Texas–(BUSINESS WIRE)–Maxwell Biosciences, a preclinical biotechnology platform (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 2, 2022
Care-Keralam, the Ayush cluster in Kerala, will shortly fine-tune its strategies to build ‘Kerala Brand of Ayurveda Products’ for international marketing and to increase the quantity of exports of traditional and herbal drugs from Kerala to global markets.

XTalks
JANUARY 29, 2021
This is relevant for clinical trials as more regulators require evidence of efficacy in comparison to the standard of care, which is likely to be one of the blockbuster products. As more trials show equivalency in outcomes between the branded and biosimilar product, more purchasers will choose the cheaper option.

Roots Analysis
MARCH 14, 2023
Clinical trials are a fundamental requirement for evaluating the safety and efficacy of novel therapeutic interventions. Initiating a clinical trial is a complex and time-consuming process, involving suitable planning and execution.
XTalks
JULY 1, 2021
Moderna has won approval from the European Medicines Association (EMA) for branding its COVID-19 vaccine with the name Spikevax. With the approval, Moderna’s Spikevax joins Pfizer-BioNTech’s Comirnaty and AstraZeneca’s Vaxzevria with European brand-name approvals. It partnered on over 75 percent of approved drug names in 2020.

Clinical Trial Podcast
JULY 18, 2020
Have you been tasked to develop a clinical trial budget? Well, you’re in luck because I’m going to share everything you need to know about clinical trial costs. Clinical trial budgets are often put together in haste. Developing a clinical trial budget can be a confusing exercise for sponsors and CROs.
Cloudbyz
MARCH 17, 2021
Monitoring patient safety during a clinical trial is one of the founding principles to be followed throughout the drug development life cycle. It can be defined as a collaborative relationship between sponsors, sites, researchers, and everyone involved in the clinical trial phases.

pharmaphorum
MAY 20, 2022
Last year, the company added clinical trials to the list with the launch of CVS Health Clinical Trial Services , an initiative that works with pharma companies on clinical trial recruitment and even hosts trial sites at certain retail locations. This interview has been edited for length and clarity.

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 17, 2024
The Department of Pharmaceuticals (DoP) has issued a new Uniform Code for Pharmaceutical Marketing Practices (UCPMP) 2024, permitting pharma companies to provide brand reminders such as informational and education items and free samples to medical professionals with restrictions on sample packs and total value.

Advarra
MARCH 21, 2023
While drugs require Phase I-III clinical trials—and are also subject to post-approval tracking—digital therapeutics, devices, and IVDs may be able to leverage bench testing, animal studies, pilot studies, and training sets.

pharmaphorum
FEBRUARY 11, 2021
Pharma companies face many challenges when involving patients in the design of clinical trials – but doing so can have huge benefits further down the line, improving the sustainability and quality of research. The post Increasing patient engagement with UK clinical trials appeared first on.

pharmaphorum
NOVEMBER 13, 2020
Where a marketing company might own and manage a domain on behalf of a pharma brand, written proof from the brand is needed to confirm collaboration with the marketing company. The post Pharma brands must ‘up the ante’ in their digital presence appeared first on. Case Study: UK leading emergency contraceptive pill.
pharmaphorum
JANUARY 12, 2022
Biogen’s difficult launch of controversial Alzheimer’s therapy Aduhelm has been made even harder by a proposal by Medicare to cover the drug only for certain patients enrolled in clinical trials. The post Medicare limits Aduhelm coverage to clinical trial participants appeared first on.

Cloudbyz
MAY 4, 2022
NAPERVILLE, Ill. , May 4, 2022 /PRNewswire/– Cloudbyz , a fast-growing integrated clinical research and development solution provider with integrated capabilities, today announced that it has partnered with Tech Mahindra to provide integrated Clinical Trial Management Solutions.
AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 17, 2023
The pill, called Opill – the brand name for the tablet formulation of norgestrel – is an oral contraceptive containing only progestin hormone, which helps prevent pregnancy […]

World of DTC Marketing
OCTOBER 21, 2021
SUMMARY: Medicare spent nearly $600 million over a three-year period to pay for four cancer drugs with no clinical benefit an analysis published Monday by JAMA Internal Medicine found. billion, with more than half of that devoted to products that did not provide a “documented overall survival benefit” in clinical trials.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 17, 2022
Acetaminophen, also known as paracetamol and sold widely under the brand names Tylenol and Panadol, also increases risk-taking, according to a study from 2020 […]. One of the most consumed drugs in the US – and the most commonly taken analgesic worldwide – could do a lot more than simply take the edge off your headache.
Pharma Mirror
SEPTEMBER 11, 2022
Bandung, W Java, Indonesia, PT Bio Farma, the holding company for state-owned pharmaceutical companies in Indonesia, announced a new milestone in the manufacturing of IndoVac, a Covid-19 vaccine brand it has developed since November 2021.

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 25, 2023
The Department of Pharmaceuticals (DoP) has rejected review applications filed by the Maharashtra-based Bharat Serums and Vaccines Ltd against National Pharmaceutical Pricing Authority (NPPA) fixing the ceiling price of its infertility treatment drug brand Hucog HP, since the company did not file the petition within the prescribed time limit.

Pharma Mirror
OCTOBER 1, 2022
Clinical Edge trains and certifies visual function examiners at investigator sites conducting Phase I to IV ophthalmic clinical trials. The combination of Optym, Emmes’ ophthalmology certification unit, and Clinical Edge will make the organization a leader in ophthalmic certification and training services.

The Pharma Data
OCTOBER 28, 2021
Bayer will present new renal and cardiovascular (CV) analyses from the comprehensive finerenone (Kerendia®) clinical trial program, including the Phase III FIGARO-DKD and FIDELIO-DKD studies, and the prespecified pooled analysis FIDELITY at the American Society of Nephrology (ASN)’s Kidney Week 2021 from 4-7 November. billion euros.

BioPharma Reporter
SEPTEMBER 3, 2020
Bluebird bio has reported end points were met in a clinical trial involving its candidate ALD cell therapy, branded as Lenti-D.
Pharmaceutical Technology
NOVEMBER 29, 2022
According to data from the ARASENS clinical trial carried out at nearly 300 sites globally, including various NHS hospitals, subjects who received the combination therapy had a 32.5% It is branded as Nubeqa and is already offered on the NHS for patients with localised prostate cancer.
Pharmaceutical Technology
MAY 1, 2023
The NDA is supported by the data package licensed to Ocumension by Nicox as well as the Phase III clinical trial in China. Zerviate, 0.24% was compared to emedastine difumarate ophthalmic solution, 0.05%, an antihistamine, which is marketed under undeb Emadine brand name.

Pharmaceutical Technology
MAY 31, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. The company’s pipeline products include Busulipo which is under Phase II clinical trials and Nefecon for IgA Nephritis which is under Phase II clinical trials.
Pharmaceutical Technology
SEPTEMBER 14, 2022
It is marketed under the brand name Covovax in the country. The latest development was based on the totality of manufacturing, preclinical and clinical trial findings filed for assessment. This data also comprises two Phase III clinical trials: a trial in the UK and PREVENT-19 in the US and Mexico.

Velocity Clinical Research
APRIL 29, 2024
Velocity’s ongoing goals are to share best practices, identify and address shortcomings, and bring as many perspectives and viewpoints to the table as possible to advance clinical trials and deliver vital new drugs and treatments to patients quicker. Show the benefits of clinical trials. Think globally, act locally.

Pharmaceutical Technology
FEBRUARY 24, 2023
The overhaul will address drug marketing exclusivity length, pricing, patient access, innovation incentives, antimicrobial resistance, clinical trials, supply chain security and shortages, and environmental impact. The EU is planning a sweeping revision of its pharma legislation in March, the largest change in 20 years.
Pharmaceutical Technology
JUNE 22, 2023
The submission of sNDA to the regulator was based on the findings obtained from the Phase III NefIgArd clinical trial. The company is also collaborating with its European commercial partner STADA Arzneimettel to seek complete approval for Nefecon, branded as Kinpeygo, from the European Commission.

Drug Discovery World
NOVEMBER 2, 2022
Norstella now has more than 1,500 employees across its five brands: Evaluate , MMIT , Panalgo , The Dedham Group , and Citeline. . “We Norstella and Citeline (formerly Pharma Intelligence) have merged to form a $5 billion global company, one of the largest pharma intelligence solutions providers on the market. .

Cloudbyz
MARCH 3, 2023
Mid-size Clinical Research Organizations (CROs) play a significant role in the pharmaceutical and biotech industry by providing various services such as study design, clinical trial management, data management, and biostatistics. Brand Awareness Mid-size CROs may lack the brand recognition that larger CROs possess.
Pharmaceutical Technology
FEBRUARY 27, 2023
Previously, the inhibitor, under the brand name Nubeqa, received approval to treat non-metastatic castration-resistant prostate cancer (nmCRPC) patients in the country. The regulatory approval is based on the data obtained from the multi-centre, double-blind, placebo-controlled, randomised Phase III ARASENS clinical trial.

XTalks
SEPTEMBER 12, 2023
By fostering a sense of community, addressing pain points and sharing impactful stories, podcasts can strengthen ties with an audience, drive brand loyalty and help life science organizations position themselves for long-term success in an increasingly competitive landscape.
World of DTC Marketing
OCTOBER 11, 2021
1ne: Don’t believe that TV alone will drive brand objectives; it’s not. They’re brighter and want to understand the data behind clinical trials better. If you plan to launch a new drug with the model of heavy-up TV and a website with some programmatic ads, you’re going to fail. 7even : Beware the PR trap.
Pharmaceutical Technology
DECEMBER 12, 2022
The latest development is based on the findings from the head-to-head Phase III PEGASUS clinical trial of Empaveli in PNH patients. The trial analysed the safety and efficacy of Empaveli versus eculizumab at 16 weeks in adult subjects with continuing anaemia despite eculizumab treatment.

Clinical Trial Gurus
AUGUST 27, 2021
As soon as we finished this recording, I made her a brand ambassador for Latinos in Clinical Research. In this video we discuss her humble beginnings as a receptionist in a clinical trial center to study coordinator to clinical research associate, and finally, to CRO owner.

Imperical Blog
JANUARY 15, 2020
In case you missed any, the following informational blogs are Imperial’s most viewed postings of 2019: Clinical Trial Branding: 5 Mistakes to Avoid Brexit, Medicines, and Trials: A Report from the U.K. The Secret to International Clinical Trial Shipments Clearing Customs.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content