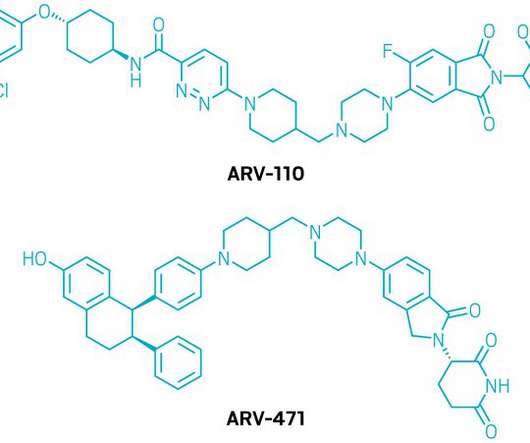
ARVINAS AND PFIZER ANNOUNCE GLOBAL COLLABORATION TO DEVELOP AND COMMERCIALIZE PROTAC® PROTEIN DEGRADER ARV-471
The Pharma Data
JULY 22, 2021
(NYSE: PFE) today announced a global collaboration to develop and commercialize ARV-471, an investigational oral PROTAC® (PROteolysis TArgeting Chimera) estrogen receptor protein degrader. The companies will equally share worldwide development costs, commercialization expenses, and profits. Chief Executive Officer at Arvinas.





















Let's personalize your content