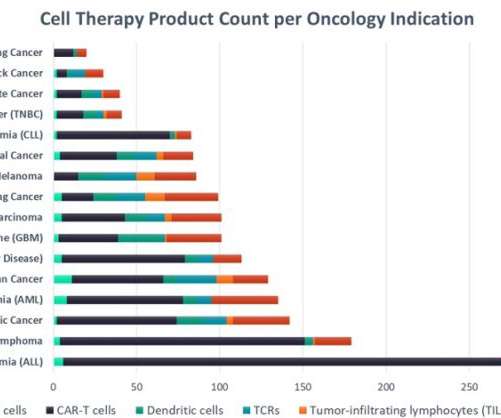
Acute lymphocytic leukaemia leads in cell therapy clinical development
Pharmaceutical Technology
JULY 12, 2022
The leading marketed CAR-T cell products, Gilead’s Yescarta (axicabtagene ciloleucel) and Novartis’ Kymriah (tisagenlecleucel), achieved $695m and $587m in sales respectively last year, demonstrating the high potential for sales of successful new cell therapies.




































Let's personalize your content