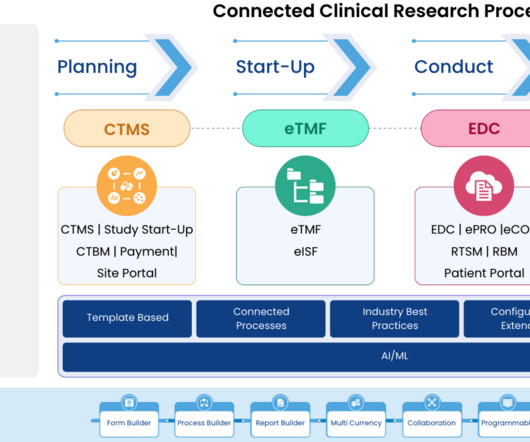
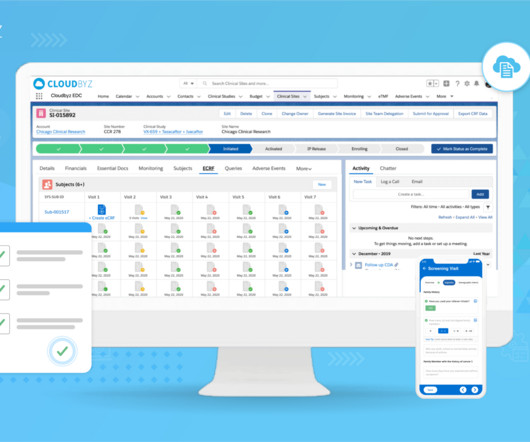
Transform Clinical Trial Management with Cloudbyz’s Unified Solution on Salesforce
Cloudbyz
APRIL 30, 2023
Scalability: Designed to scale with the needs of its users, Cloudbyz’s platform can accommodate clinical trials of varying sizes and complexities. This ensures that the solution remains useful and valuable as organizations grow and evolve, meeting the ever-changing demands of the clinical research landscape.










Let's personalize your content