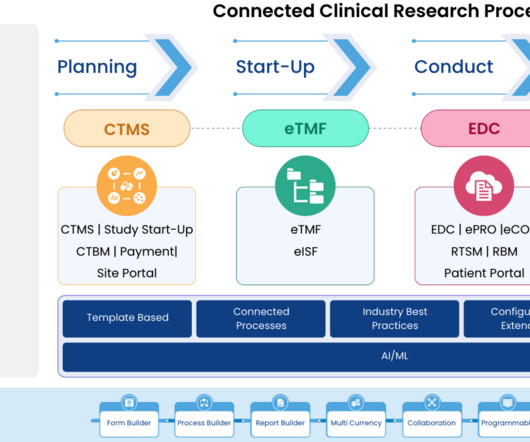
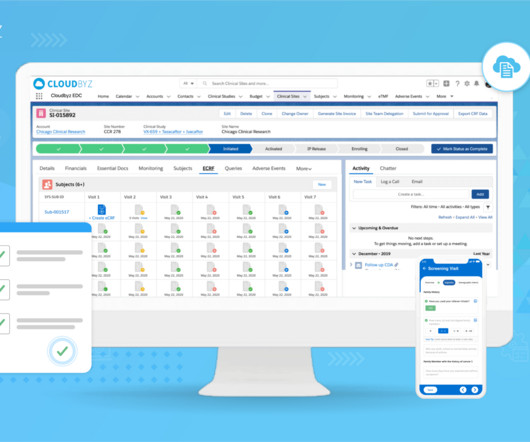
Transform Clinical Trial Management with Cloudbyz’s Unified Solution on Salesforce
Cloudbyz
APRIL 30, 2023
Integration with Third-Party Tools: Cloudbyz’s solution can integrate with a variety of third-party tools and applications, further enhancing its capabilities and streamlining the clinical trial management process.












Let's personalize your content