Clinical Trial Manager Jobs: What You Should Know
XTalks
MAY 9, 2023
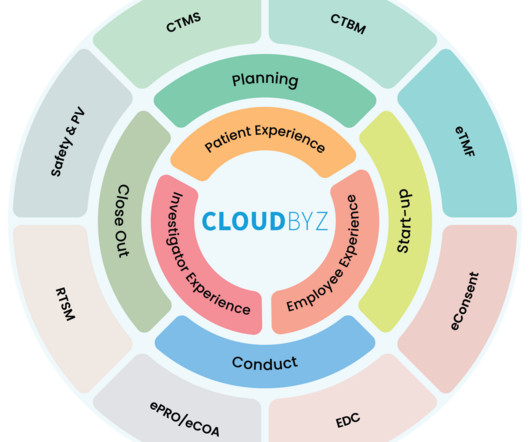
Clinical trial manager jobs are vital for the development and execution of clinical trials, which are essential for developing new treatments for diseases. In this article, we discuss the job duties, education and experience requirements, outlook and salary expectations for clinical trial managers.





























Let's personalize your content