CDER’s Office of Compliance Kicks Off Annual Report Season with Rundown of 2023’s Major Public Health Enforcement Initiatives
FDA Law Blog
JANUARY 22, 2024
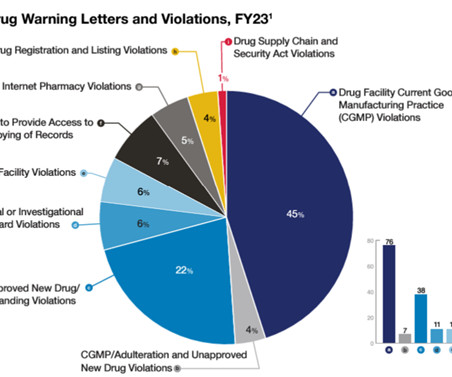
Claud — The Office of Compliance (OC) at FDA’s Center for Drug Evaluation and Research (CDER) had a role in many of the major public health enforcement matters you may have read about last year. OC devoted a lot of resources to its compliance efforts directed at human drug compounding. By John W.M. Clinical Trials.




















Let's personalize your content