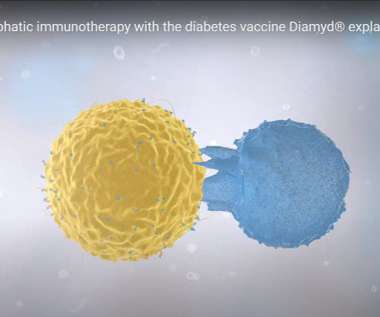
Type 1 Diabetes Vaccine: Does Diamyd Have a Winning Formula?
XTalks
SEPTEMBER 15, 2020
In addition to the vaccine, the company is also developing an investigational drug called Remygen for the regeneration of endogenous insulin production and to improve hormonal response to hypoglycemia; trials to test the treatment are currently ongoing in patients that have been living with type 1 diabetes for more than five years.









Let's personalize your content