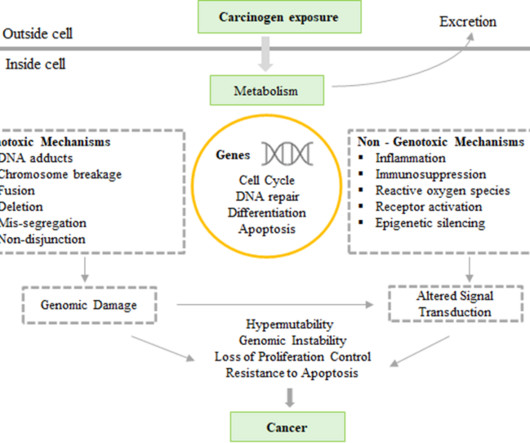
#news #biotech PCR-test reveals risk of genotoxity of genome editing
BioTech 365
MARCH 10, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: PCR-test reveals risk of genotoxity of genome editing. from European Biotechnology – first and foremost in European biotech [link].























Let's personalize your content