BioSig abandons COVID-19 drug trial on safety concerns
pharmaphorum
OCTOBER 27, 2020
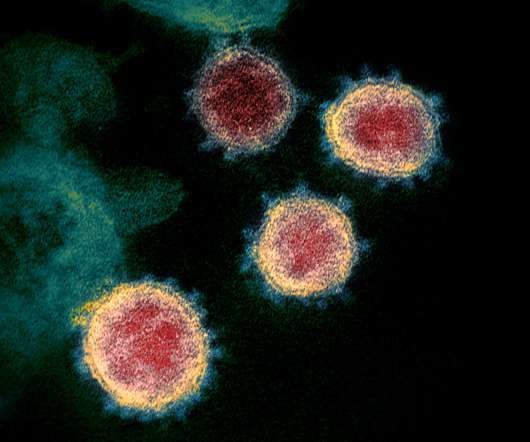
US biotech BioSig Technologies has abandoned a phase 2 trial of its antiviral drug merimepodib with Gilead’s Veklury in severe COVID-19 patients, after concluding the safety of the combination was in doubt. Moreover, as an orally-active drug merimepodib could also have found a role outside the hospital setting.













Let's personalize your content