Chinese manufacturers’ transition to innovative pharma requires more investment
Pharmaceutical Technology
NOVEMBER 22, 2022
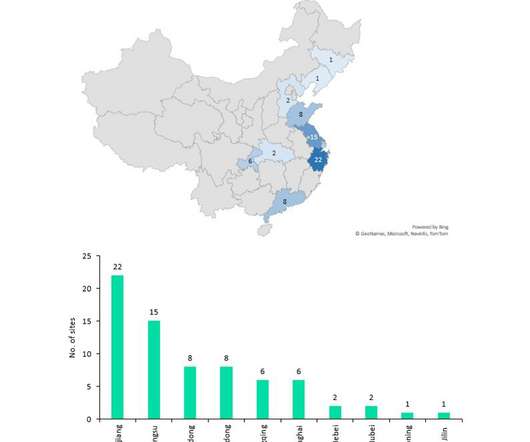
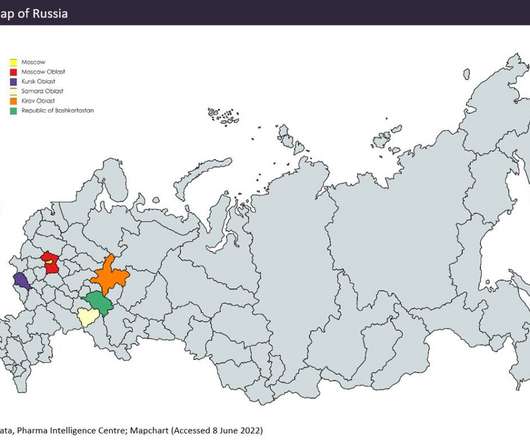
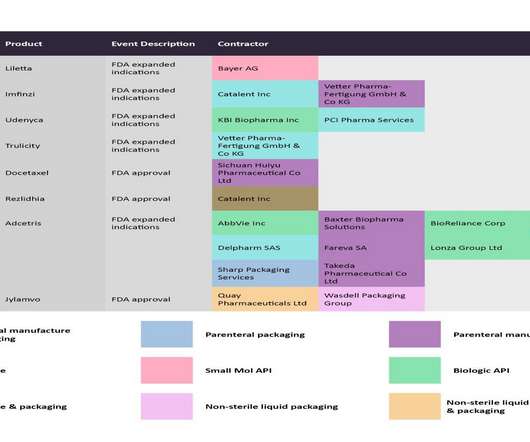
Despite China producing a significant proportion of the world’s API supply (mostly small molecule), it manufactures relatively few biosimilar and innovator drugs and no cell and gene therapies for the western markets of Europe and the US despite investments and an increasing number of startups to improve innovative manufacture.











Let's personalize your content