FDA Warns About Compounded Versions of Ozempic and Wegovy
XTalks
JUNE 5, 2023
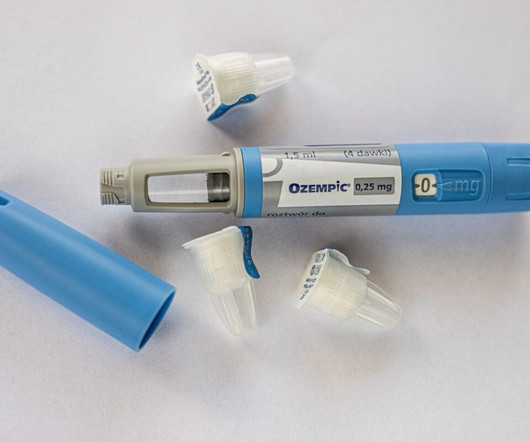
GLP-1 stimulates insulin production, thus reducing blood glucose levels, and it interacts with the brain to suppress appetite and create a feeling of fullness. Novo Nordisk, the pharmaceutical manufacturer of Ozempic and Wegovy, is actively addressing these supply challenges. The approved drugs contain the base form of semaglutide.












Let's personalize your content