LNP formulations produce strong immune responses, data shows
Drug Discovery World
FEBRUARY 15, 2024
Hypothetically, any mRNA-based drug using S-Ac7-DOG as the lipid base would therefore have improved efficacy and a better safety profile.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Drug Discovery World
FEBRUARY 15, 2024
Hypothetically, any mRNA-based drug using S-Ac7-DOG as the lipid base would therefore have improved efficacy and a better safety profile.
Pharma Mirror
OCTOBER 16, 2023
The first mRNA drug (BNT162b2 vaccine) was granted emergency use authorization by the FDA in December 2020 and approved for marketing in August 2021. The first mRNA drug (BNT162b2 vaccine) was granted emergency use authorization by the FDA in December 2020 and approved for marketing in August 2021.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JUNE 23, 2022
The US Food and Drug Administration (FDA) has granted approval for Merck's (MSD outside the US and Canada) pneumococcal 15-valent conjugate vaccine, Vaxneuvance, for use in children aged six weeks to 17 years. The post US FDA grants approval for Merck’s pneumococcal vaccine for children appeared first on Pharmaceutical Technology.
Drug Discovery World
MAY 2, 2024
A study led by the University of Oxford has successfully investigated human immunity against Covid-19 in people who already have antibodies, with the aim of advancing future vaccines and treatments. The results suggest that previous infection, together with vaccination, offers strong protection against the original Covid-19 strain.
Drug Discovery World
JANUARY 16, 2024
A new study will compare whether giving tuberculosis vaccine by inhalation is better at protecting against tuberculosis (TB) than injection into the skin. The Jenner Institute at the University of Oxford is conducting the study using Bacille Calmette-Guérin (BCG), the current licensed vaccine against TB.
Pharmaceutical Technology
NOVEMBER 20, 2022
Health Canada has granted expanded authorisation for Novavax ’s Covid-19 vaccine (Recombinant protein, Adjuvanted) [Nuvaxovid; NVX-CoV2373] as a homologous booster for usage in adults aged 18 and above. The vaccine is indicated for active immunisation to prevent the disease.

Pharmaceutical Technology
JUNE 10, 2023
Influenza vaccine 2 is under clinical development by Shanghai Institute of Biological Products and currently in Phase I for Pandemic Influenza. According to GlobalData, Phase I drugs for Pandemic Influenza does not have sufficient historical data to build an indication benchmark PTSR for Phase I. Buy the report here.

Pharmaceutical Technology
JUNE 10, 2023
Influenza vaccine 2 is under clinical development by Shanghai Institute of Biological Products and currently in Phase I for Pandemic Influenza. According to GlobalData, Phase I drugs for Pandemic Influenza does not have sufficient historical data to build an indication benchmark PTSR for Phase I. Buy the report here.
pharmaphorum
OCTOBER 11, 2022
An intranasal formulation of AstraZeneca’s widely-used COVID-19 vaccine Vaxzevria has failed at the first hurdle, after results from a phase 1 trial found it was unable to stimulate a strong immune response to the virus. Other efforts to develop a nasally-delivered COVID-19 vaccine have met with greater success.

NY Times
MAY 11, 2022
Antibody levels rose in the children who received it, suggesting the vaccine protects against infection. But the data were gathered before the arrival of Omicron.
Pharmaceutical Technology
APRIL 11, 2023
The US Food and Drug Administration (FDA) has granted final approval to India-based Zydus Lifesciences ‘ 500mg azithromycin tablets for the treatment of bacterial infections. Zydus will manufacture the drug at its formulation facility in Moraiya, Ahmedabad, in the Indian state of Gujarat.

Pharmaceutical Technology
JUNE 29, 2022
The Japanese Ministry of Health, Labour and Welfare (MHLW) has accepted GlaxoSmithKline’s (GSK) regulatory application for recombinant, adjuvanted Zoster vaccine, Shingrix, for preventing shingles (herpes zoster) in at-risk adults of the age 18 years and above.
Drug Discovery World
FEBRUARY 19, 2024
Replicate Bioscience has shared positive results from the Phase I trial of RBI-4000, its srRNA-based rabies vaccine. In this clinical trial, evaluating safety and immunogenicity of RBI-4000 , participants received one or two doses of srRNA vaccine at low doses (0.1mcg, 1mcg or 10mcg).

Scienmag
SEPTEMBER 21, 2020
September 21, 2020–(BRONX, NY)–The first study comparing the immune responses of adults and children with COVID-19 has detected key differences that may contribute to understanding why children usually have milder disease than adults. The study was published today in Science […].
Drug Discovery World
APRIL 26, 2024
A clinical trial of a personalised mRNA cancer vaccine for melanoma patients has been launched in the UK. In addition to encoding the target antigens, mRNA vaccines also provide adjuvant properties that amplify the immune response. in the combination arm and 62.2%

Drug Discovery World
FEBRUARY 20, 2024
A five-year contract totalling up to $31 million including programme options has been awarded to Ginkgo Bioworks to discover and develop next-generation vaccine adjuvants. The post Ginkgo Bioworks to discover and develop novel vaccine adjuvants appeared first on Drug Discovery World (DDW).
NY Times
MARCH 23, 2022
The company said the vaccine produced a strong immune response in children younger than 6, but proved only about 40 percent effective in preventing symptomatic Covid-19.
Drug Discovery World
AUGUST 3, 2023
It becomes the ninth Covid-19 vaccine to be authorised by the UK’s independent medicines regulator. The clinical evidence for the authorisation is based on data from a study of 765 adults who had received primary vaccination with two doses of the Comirnaty Covid-19 vaccine and who were given a booster dose of either Bimervax or Comirnaty.
pharmaphorum
JUNE 30, 2021
Just a few months after starting clinical trials of its nasal spray vaccine for COVID-19, US biotech Altimmune is abandoning the project, saying that it generated weaker than expected immune responses in a phase 1 trial. .
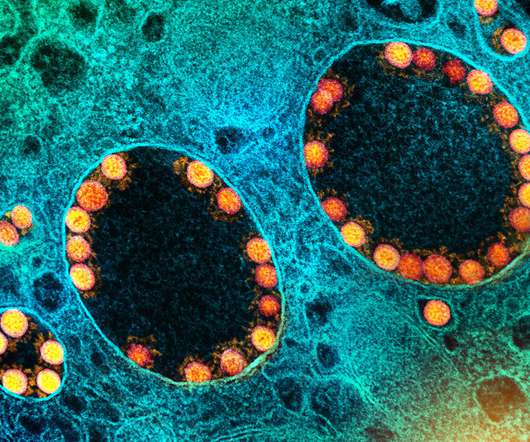
Scienmag
JANUARY 26, 2022
WHAT: In adults who had previously received a full regimen of any of three COVID-19 vaccines granted Emergency Use Authorization (EUA) or approved by the Food and Drug Administration (FDA), an additional booster dose of any of these vaccines was safe and prompted an immune response, according to preliminary clinical trial results reported in The […]. (..)
Pharmaceutical Technology
NOVEMBER 11, 2022
The European Commission (EC) has granted approval for Sanofi and GSK ’s monovalent, recombinant-protein-based, adjuvanted Covid-19 vaccine, VidPrevtyn Beta, as a booster in adults aged 18 years and above. It is indicated as a booster in people of this age group who were earlier inoculated with a Covid-19 vaccine.
World of DTC Marketing
AUGUST 3, 2020
QUICK READ: As COVID-19 vaccines enter Phase III trials one has to wonder just how transparent pharma companies will be with the data. Personally I would want to know as much as I can before I ask for the vaccine but I also know that greed can make people do stupid things and there is a lot of money at stake here.
Pharmaceutical Technology
AUGUST 26, 2022
Amid a shortage of monkeypox vaccine doses, the WHO and FDA have declared public health emergencies, and the FDA has granted its first Emergency Use Authorization (EUA) to a monkeypox vaccine and opened the door for more. On the same day, the FDA granted a EUA to Bavarian Nordic’s (Copenhagen, Denmark) Jynneos vaccine.
Drug Discovery World
MARCH 30, 2023
Likang Life Sciences has been granted implied approval by China’s National Medical Products Administration (NMPA) for the clinical trial of its innovative personalised neoantigen-targeted vaccine LK101 Injection for advanced solid tumours. Vaccines predicated on neoantigens therefore elicit truly tumour-specific T cell responses.
Drug Discovery World
SEPTEMBER 15, 2023
Replicate Bioscience has dosed the first participant in a Phase I trial of its RBI-4000 vaccine for the prevention of rabies. Replicate’s srRNA technology offers the potential for more robust and durable immune responses, and improved tolerability at lower doses than existing mRNA approaches,” said Zelanna Goldberg, CMO of Replicate. “In
Pharmaceutical Technology
APRIL 10, 2023
Health director-general Tan Sri Dr Noor Hisham Abdullah stated that the approval of Evusheld was granted at the 383rd Drug Control Authority (DCA) meeting. The approval comes after the therapy was granted conditional registration approval during the 372nd DCA meeting.

pharmaphorum
FEBRUARY 9, 2022
In this article, Ben Hargreaves looks into the promise of cancer vaccines and how this treatment modality may offer advantages over existing immunotherapies in the oncology sector. One area that is gathering increasing levels of interest is the development of cancer vaccines. over the last five years. A broad front.
Drug Discovery World
OCTOBER 31, 2023
The Oxford Vaccine Group at the University of Oxford, UK, has launched the LEGACY03 study into human immunity, aimed to improve the design of seasonal vaccines and allow targeting by age group. As we age, our immune system changes and with it our response to vaccines.
pharmaphorum
JUNE 21, 2021
It would be easy to forget that back in 2019, BioNTech was an early-stage biotech firmly focused on cancer vaccines, before being catapulted onto the world-stage with its COVID-19 shot. Efficacy was assessed in a subset of 42 patients treated with the shot as a monotherapy or alongside an anti-PD-1 drug.
Drug Discovery World
OCTOBER 13, 2023
The scientists say the tool can help inform the development of vaccines and therapies for SARS-CoV-2 and other rapidly mutating viruses. The University has also launched a new global consortium to research and develop next-generation Covid-19 and flu vaccines, backed by £8 ($9.8) million funding from UK Research and Innovation (UKRI).
Drug Discovery World
SEPTEMBER 25, 2023
Enrolment in a Phase I trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland, US. An ideal universal influenza vaccine could be taken less frequently than once a year and protect against multiple strains of influenza virus. “An
pharmaphorum
OCTOBER 2, 2020
Roche’s Genentech unit has paid $200 million to develop an individualised cancer vaccine with Norwegian biotech Vaccibody, which focuses on targets known as neoantigens that spring up as tumours grow and mutate. Frame Therapeutics has also begun work on neoantigen-based cancer vaccines based on RNA technology.

pharmaphorum
DECEMBER 6, 2022
Pfizer and partner BioNTech have launched a countersuit against Moderna in a patent despite centring on their mRNA-based vaccines for COVID-19. It also waived its rights to bring the lawsuit when it pledged not to sure other COVID-19 vaccine producers during the pandemic, according to Reuters.

pharmaphorum
JULY 6, 2021
A peptide vaccine developed by Australia’s Imugene has reduced tumour size in around half of patients with HER2-positive gastric or gastroesophageal junction (GEJ) cancer in a phase 2 trial. ” The anti-HER2 market is advancing rapidly however, with a new generation of drugs coming through the industry pipeline.
Drug Discovery World
MAY 18, 2023
A clinical trial of an experimental universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC) has begun enrolling volunteers at Duke University in Durham, North Carolina. The trial will enrol up to 50 healthy volunteers aged 18 to 49.
XTalks
JUNE 24, 2022
The US Food and Drug Administration (FDA) has authorized the use of Pfizer-BioNTech’s and Moderna’s COVID-19 vaccines for children as young as six months old, a much-awaited decision for some parents with young children. The vaccine had been authorized for use in adults 18 years of age and older. Califf, MD.

XTalks
SEPTEMBER 5, 2023
Vaccines have been an integral piece of the global public health toolbox for over 200 years, but the COVID-19 pandemic brought about a new era in vaccine development with renewed interest in mRNA technology and unprecedented accelerated regulatory approvals. What are the major challenges you’re facing right now in vaccine development?

pharmaphorum
AUGUST 25, 2022
Today on the pharmaphorum podcast, Editor in Chief Jonah Comstock welcomes Hedi Ben Brahim and Eric Quéméneur, CEO and chief science officer respectively of Transgene, to discuss cancer vaccines in general and Transgene’s recent work in the space in particular. . Podbean. .

XTalks
APRIL 26, 2021
After years of disappointing malaria vaccine trials, a malaria shot developed by researchers at the Jenner Institute at the University of Oxford has demonstrated an unprecedentedly high efficacy of 77 percent, and may be the magic bullet the world has been waiting for against the deadly disease.
Drug Discovery World
OCTOBER 27, 2023
HB-400, a novel arenaviral therapeutic vaccine developed by HOOKIPA Pharma and Gilead Sciences, has been shown to generate robust T cell responses specific to hepatitis B virus with high antibody levels in a preclinical setting. A Phase I clinical trial to evaluate the safety and tolerability of HB‑400 in humans is ongoing.
Pharmaceutical Technology
JUNE 21, 2023
The Drug Controller General of India (DCGI) has granted emergency use authorisation (EUA) for Gennova Biopharmaceuticals’ Omicron-specific mRNA-based Covid-19 booster vaccine, GEMCOVAC-OM. The vaccine produced significantly higher immune responses when given as a booster.
NY Times
APRIL 14, 2022
Pfizer and BioNTech will soon ask the Food and Drug Administration for emergency authorization of Covid booster doses for that age group, the companies said.
XTalks
JULY 28, 2021
Merck has scored US Food and Drug Administration (FDA) approval for its next-generation pneumonia vaccine Vaxneuvance that covers 15 different strains of the pneumococcal bacteria that causes the infection. This is seven more strains than its current winning vaccine Prevnar 13, which registered $5.95

Drug Discovery World
JANUARY 12, 2024
This week has seen a number of significant discoveries by researchers in academic institutions, in some cases in partnership with industry, emphasising the important role universities play in early-stage drug discovery. The post This week in drug discovery (8-12 January) appeared first on Drug Discovery World (DDW).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content