Bristol Myers CAR-T therapy approved by FDA for earlier myeloma use
Bio Pharma Dive
APRIL 5, 2024
The FDA’s clearance comes three weeks after a panel of advisers endorsed expanded use of Abecma despite safety concerns raised by the agency.

Bio Pharma Dive
APRIL 5, 2024
The FDA’s clearance comes three weeks after a panel of advisers endorsed expanded use of Abecma despite safety concerns raised by the agency.

Pharmaceutical Technology
APRIL 5, 2024
MiNA Therapeutics has entered into an agreement with Nippon Shinyaku to develop RNAa therapeutics for rare neurodegenerative ailments.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
APRIL 5, 2024
The German pharmaceutical company is “streamlining” its custom-facing teams behind Cyltezo, a copycat version of AbbVie’s immune disease drug Humira.

Worldwide Clinical Trials
APRIL 5, 2024
For many people, February 29th is simply Leap Day, an extraordinary date that appears only once every four years. For others, this rare day is aptly the day we honor rare disease communities. Observed annually on the last day of February since 2008, Rare Disease Day has grown into a global movement for raising awareness, promoting research, and advocating for improved access to rare disease treatments and support services.

Pharmaceutical Technology
APRIL 5, 2024
The director of BARDA’s medical countermeasures programs, Robert Johnson, talks about what the agency is planning to invest in the future.

Fierce Pharma
APRIL 5, 2024
After a short period of doubt, the FDA has followed the opinion of its advisers and moved Bristol Myers Squibb’s CAR-T therapy Abecma into the earlier treatment of multiple myeloma. | After a short period of doubt, the FDA has followed the opinion of its advisers and moved Bristol Myers’ CAR-T therapy Abecma into the earlier treatment of multiple myeloma.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Fierce Pharma
APRIL 5, 2024
Vertex CEO Reshma Kewalramani, M.D., made $20.6 million in 2023, which was an increase of 30% from her $15.9 million pay in 2022.

Pharmaceutical Technology
APRIL 5, 2024
Last month, Amylyx announced that the Phase III confirmatory trial of ALS drug Relyvrio failed to meet its primary and secondary endpoints.
Pharma Times
APRIL 5, 2024
Hoxa9 and b-catenin molecules are a rare population of self-renewing HSCs found in bone marrow

Pharmaceutical Technology
APRIL 5, 2024
Acumen has entered a partnership with Lonza to further the development of sabirnetug, a potential treatment for Alzheimer's disease.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
APRIL 5, 2024
The deal comes just over a week after The Wall Street Journal reported the companies were discussing a merger.

Pharmaceutical Technology
APRIL 5, 2024
Despite a failed bid to expand Imfinzi’s treatment scope in NSCLC, the drug has proved effective in treating small cell lung cancer.

BioSpace
APRIL 5, 2024
The American Association for Cancer Research (AACR)’s annual conference runs April 5 through April 10. BioSpace will have all the key data and news out of San Diego.

Pharmaceutical Technology
APRIL 5, 2024
Caris Life Sciences has entered a collaboration with Merck KGaA for the development of antibody-drug conjugates (ADCs) for cancer.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Commerce
APRIL 5, 2024
In an interwith with Pharma Commerce Editor Nicholas Saraceno, Ed Schoonveld, Value and Access Advisor, Schoonveld Advisory, explains why pharmaceutical products tend to cost more in the US than the rest of the world.
Pharmaceutical Technology
APRIL 5, 2024
The UK MHRA has approved Advanz Pharma’s combined antibiotic, cefepime/enmetazobactam, to treat complicated infections.

Fierce Pharma
APRIL 5, 2024
AstraZeneca's Imfinzi has delivered positive clinical data in another type of lung cancer, building on the immunotherapy’s existing FDA clearance in stage 3 non-small cell lung cancer and extensive | AstraZeneca's Imfinzi has delivered positive clinical data in another type of lung cancer, building on the immunotherapy’s existing FDA clearance in stage 3 non-small cell lung cancer and extensive-stage small cell lung cancer.

Pharmaceutical Technology
APRIL 5, 2024
GSK’s Trelegy Ellipta has been approved as a maintenance therapy for both asthma and COPD in Singapore.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

XTalks
APRIL 5, 2024
Amylyx Pharmaceuticals announced yesterday that it will be pulling its ALS (amyotrophic lateral sclerosis) drug Relyvrio from the market after failing in a confirmatory trial. In a statement , Amylyx said it has initiated a process with the US Food and Drug Administration (FDA) and Health Canada to voluntarily discontinue the marketing authorizations for Relyvrio.

Fierce Pharma
APRIL 5, 2024
Amid ongoing drug shortages and other headline-grabbing issues that fall under the FDA’s purview, the House Committee on Oversight and Accountability is putting the agency’s commissioner Robert Cal | The April 11 hearing could be contentious, as the committee wants to “hold the commissioner accountable" for the FDA's action on drug shortages, among other issues, according to a statement from Chairman James Comer, R-Kentucky.

pharmaphorum
APRIL 5, 2024
Our regular round-up of financings in the biotech sector is headed by rumours of a $150 to $200 million initial public offering (IPO) for Aardvark Therapeutics, an emerging player in the weight-loss category, alongside a trio of big private rounds.

Fierce Pharma
APRIL 5, 2024
Despite Boehringer Ingelheim’s 2021 Humira biosimilar approval being heralded by some analysts as a “landmark achievement” for the field, the drug—dubbed Cyltezo—has struggled to gain traction than | Facing lackluster Cyltezo sales, Boehringer Ingelheim will prune its ranks in the U.S. and adopt a different way of marketing its Humira biosimilar.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
pharmaphorum
APRIL 5, 2024
Advances in immunosuppression research may bring transplant patients closer to “one organ for life” Mike.

Fierce Pharma
APRIL 5, 2024
After Novo Holdings set the stage for a $16.5 billion buyout of contract manufacturing giant Catalent earlier this year, its parent company is giving antitrust officials more time for their review | After Novo Holdings pitched a $16.5 billion buyout of Catalent in February, the companies have given the FTC extra time to review the deal.
pharmaphorum
APRIL 5, 2024
ADRIATIC trial finds AstraZeneca's cancer immunotherapy Imfinzi improves survival in patients with limited-stage small cell lung cancer (SCLC)

BioSpace
APRIL 5, 2024
Contineum Therapeutics priced its initial public offering Friday, scaling back its expectations for gross proceeds of $110 million for clinical trials of a challenger to Boehringer Ingelheim and Roche.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Cloudbyz
APRIL 5, 2024
Implementing Electronic Data Capture (EDC) systems in clinical trials offers numerous benefits, including improved data accuracy, faster data availability, and streamlined processes. However, biotechnology sponsors face several practical challenges during its implementation. Here are ten of these challenges along with best practices to avoid or mitigate them: 1.

BioSpace
APRIL 5, 2024
The FTC and the U.S. Department of Justice’s antitrust division will have another 30 days to examine Novo Nordisk Foundation’s acquisition of contract manufacturer Catalent, according to an SEC filing.
Pharma Times
APRIL 5, 2024
Affecting 80,000 people in England, 12% of brain tumour patients survive beyond five years of diagnosis

BioSpace
APRIL 5, 2024
Analysts and attendees aren’t expecting groundbreaking data at the American Association for Cancer Research’s annual conference this year, but for many, that isn’t the point.
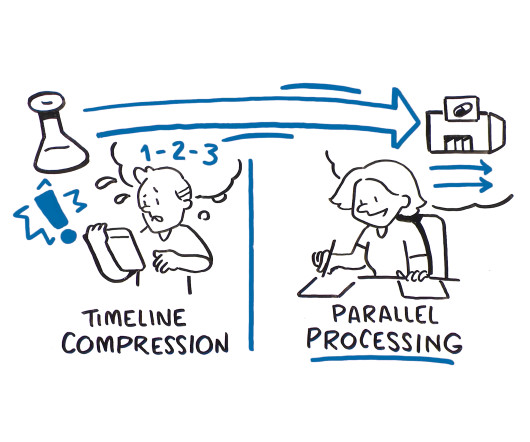
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content