JURA Bio and Syena partner to develop TCR-based therapies
Pharmaceutical Technology
SEPTEMBER 22, 2023
JURA Bio has entered into a research collaboration with Replay cell therapy product company Syena for the development of TCR based therapies.

Pharmaceutical Technology
SEPTEMBER 22, 2023
JURA Bio has entered into a research collaboration with Replay cell therapy product company Syena for the development of TCR based therapies.
Bio Pharma Dive
SEPTEMBER 22, 2023
Results show that the medicine helped patients who progressed on earlier-line treatments live longer than those receiving chemotherapy without their disease getting worse, according to a Friday announcement.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
SEPTEMBER 22, 2023
A Phase I trial exploring the gene therapy in patients with alpha-1 antitrypsin deficiency is expected to start in Q1 2024.

Bio Pharma Dive
SEPTEMBER 22, 2023
The drug, which is already cleared as a neoadjuvant lung cancer treatment, could further compete with Merck’s Keytruda if it gains approval in the adjuvant setting for patients with the non-small cell form of the disease.

Pharmaceutical Technology
SEPTEMBER 22, 2023
According to GlobalData, the number of individuals affected by rheumatoid arthritis is anticipated to rise to 6,980,823 cases by 2029.
Bio Pharma Dive
SEPTEMBER 22, 2023
Compared to chemotherapy, a combination of Seagen’s Padcev and Merck’s Keytruda was significantly better at keeping bladder cancer patients alive longer and their disease from progressing.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Fierce Pharma
SEPTEMBER 22, 2023
Merck and Eisai’s Keytruda-Lenvima tag team can’t seem to catch a break. | Merck and Eisai’s Keytruda-Lenvima tag team can’t seem to catch a break. Following a series of trial shortfalls this year in melanoma, colorectal cancer and head and neck squamous cell carcinoma, the duo has missed the mark in yet another set of clinical trials—this time in non-small cell lung cancer.
Pharmaceutical Technology
SEPTEMBER 22, 2023
The European Commission has approved Enrylaze for treating acute lymphoblastic leukaemia and lymphoblastic lymphoma.

Antidote
SEPTEMBER 22, 2023
Connecting patients with clinical research opportunities is our mission here at Antidote, but often, we find that misconceptions can serve as barriers to achieving this goal. It’s important to carefully weigh the pros and cons of participating in a clinical trial but to do this effectively, it’s critical to have accurate information.
Pharmaceutical Technology
SEPTEMBER 22, 2023
Debiopharm and SunRock Biopharma have signed an agreement to advance the development of specifically targeted ADCs for hard-to-treat cancers.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Fierce Pharma
SEPTEMBER 22, 2023
Fast on the heels of winning a European approval to treat chronic kidney disease (CKD), Eli Lilly and Boehringer Ingelheim's versatile SGLT2 inhibitor Jardiance is expanding its reach in the U.S., | After AstraZeneca's Farxiga won a class-first chronic kidney disease approval back in 2021, Eli Lilly and Boehringer are entering the fray with their SGLT2 drug Jardiance.

Pharmaceutical Technology
SEPTEMBER 22, 2023
The WHO and the World Bank have published the 2023 UHC Global Monitoring Report, detailing global health spending and service coverage rate.

Fierce Pharma
SEPTEMBER 22, 2023
It’s not a proper market showdown without all the players. | It’s not a proper market showdown without all the players. In the perioperative treatment of early-stage non-small cell lung cancer with a PD-1/L1 inhibitor, Bristol Myers Squibb is touting a new trial win. The results follow positive readouts from AstraZeneca and Merck.

Pharmaceutical Technology
SEPTEMBER 22, 2023
The UK-based surgical robotics company has now raised over $1bn in funding, cementing its unicorn status as it passes 15,000 surgeries.

Fierce Pharma
SEPTEMBER 22, 2023
As Seagen prepares for its takeover by Pfizer, the company’s Astellas-partnered cancer drug Padcev has chalked up a key trial win—in combination with Merck's Keytruda—in previously untreated metast | In a critical win, the Padcev-Keyturda combo significantly reduced the risk of death in first-line bladder cancer patients who are eligible for platinum-based chemotherapy at an interim analysis of the closely watched EV-302 trial.

Pharmaceutical Technology
SEPTEMBER 22, 2023
Glenmark Pharmaceuticals has signed a definitive agreement for the divestiture of a 75% stake in Glenmark Life Sciences for $679.85m.

Pharma Times
SEPTEMBER 22, 2023
The collaboration will utilise Evaxion’s EDEN-discovered antigen targets - News - PharmaTimes

Pharmaceutical Technology
SEPTEMBER 22, 2023
Novo Holdings has acquired Paratek Pharmaceuticals for nearly $462m to bolster its antimicrobial resistance (AMR) expertise.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Commerce
SEPTEMBER 22, 2023
An analysis by 3 Axis Advisors has found that there is a large variability in pharmacy reimbursement of prescription drugs depending on PBM contracts with insurers. This creates a system with huge inconsistencies on the prices of both generics and branded products.

Pharma Times
SEPTEMBER 22, 2023
IDAP aims to address unmet clinical needs for patients and healthcare professionals - News - PharmaTimes
Drug Channels
SEPTEMBER 22, 2023
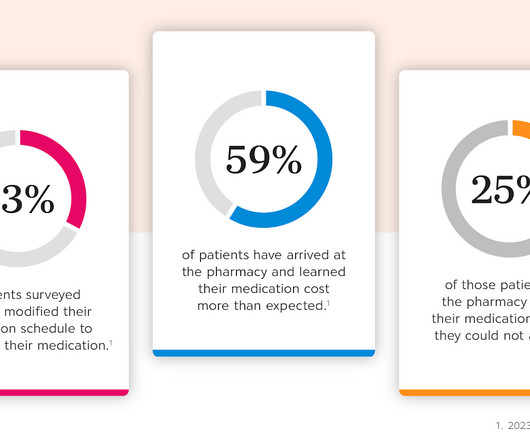
Today’s guest post comes from Kristina Crockett, VP of Product Management at CoverMyMeds. Kristina shares the story of a patient who cut pills in half to extend her prescriptions. Unfortunately, this patient didn’t know about manufacturer affordability programs. In this article, Kristina reviews how brands can help patients make connections that address medication affordability challenges, whether at the prescriber’s office, the pharmacy, or at home.

Fierce Pharma
SEPTEMBER 22, 2023
A story this week about an FDA Form 483 filing for an Ajinomoto site in San Diego was reported in error. While the site did receive FDA observations in early 2023, the inspection has since closed. | A story published Sept. 19 about an Ajinomoto site in San Diego has been retracted.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Drug Channels
SEPTEMBER 22, 2023
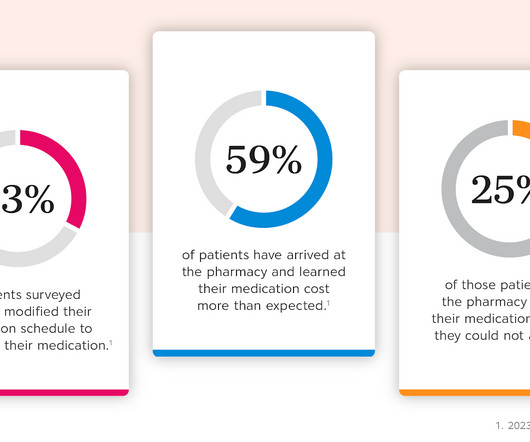
Today’s guest post comes from Kristina Crockett, VP of Product Management at CoverMyMeds. Kristina shares the story of a patient who cut pills in half to extend her prescriptions. Unfortunately, this patient didn’t know about manufacturer affordability programs. In this article, Kristina reviews how brands can help patients make connections that address medication affordability challenges, whether at the prescriber’s office, the pharmacy, or at home.

pharmaphorum
SEPTEMBER 22, 2023
Machine learning puts virtual drug screening into high gear Phil.

XTalks
SEPTEMBER 22, 2023
Recent initial public offerings (IPOs) from food companies such as Cava Group and BranchOut Food might give the impression of a reawakening IPO market after a seemingly quiet year. However, EY’s latest Global IPO Trends report for the second quarter of 2023 suggests otherwise. While a few IPOs have captured media attention, the broader US IPO scene remains muted.

pharmaphorum
SEPTEMBER 22, 2023
Seagen hails ‘practice-changing’ trial in bladder cancer Phil.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Drug Patent Watch
SEPTEMBER 22, 2023
Annual Drug Patent Expirations for ACTHAR+GEL Acthar Gel is a drug marketed by Mallinckrodt Ard and is included in one NDA. It is available from one supplier. The generic ingredient… The post New patent for Mallinckrodt Ard drug ACTHAR GEL appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
SEPTEMBER 22, 2023
AZ, Daiichi Sankyo cue up TROP2 drug filing in breast cancer Phil.

Drug Patent Watch
SEPTEMBER 22, 2023
Annual Drug Patent Expirations for BYLVAY Bylvay is a drug marketed by Albireo and is included in one NDA. It is available from one supplier. There are eight patents protecting… The post New patent for Albireo drug BYLVAY appeared first on DrugPatentWatch - Make Better Decisions.
pharmaphorum
SEPTEMBER 22, 2023
Travere weaker after Filspari fails confirmatory trial Phil.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content