On heels of FDA approval, trial results set up GSK's RSV vaccine for label expansion
Fierce Pharma
OCTOBER 25, 2023
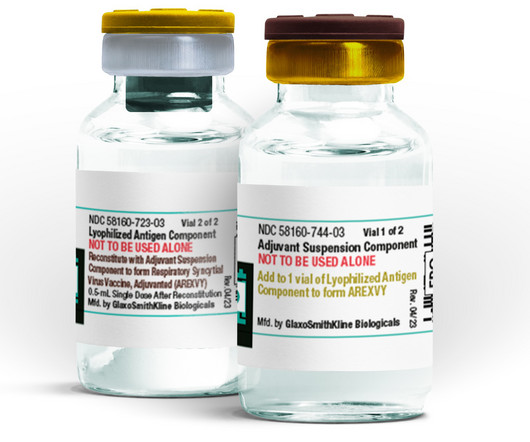
Five months after becoming the first company to secure FDA approval for a respiratory syncytial virus (RSV) vaccine, GSK is taking steps toward expanding its label for Arexvy.































Let's personalize your content