US FDA approves Bausch + Lomb and Novaliq’s DED treatment Miebo
Pharmaceutical Technology
MAY 19, 2023
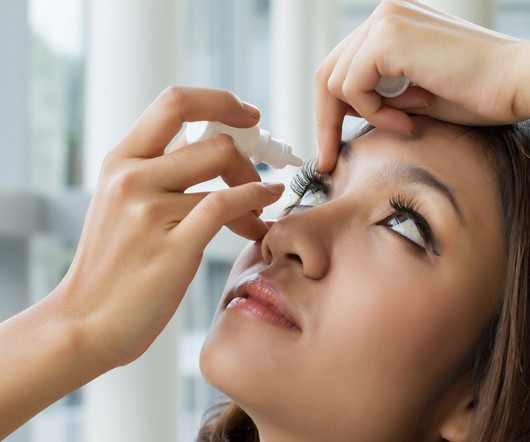
It is the first and only prescription eye drop to receive FDA approval to treat DED that targets tear evaporation directly. Bausch + Lomb chairman and CEO Brent Saunders stated: “Today’s FDA approval of Miebo further advances DED treatment by addressing a significant unmet need for millions of people suffering with this disease. “We




































Let's personalize your content