‘Proactive’ vaccine can protect against unknown future coronaviruses
Drug Discovery World
MAY 15, 2024
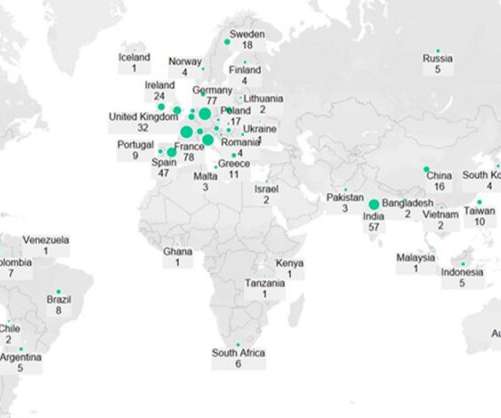
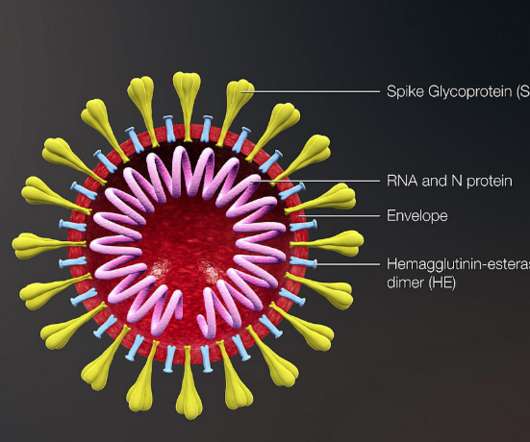
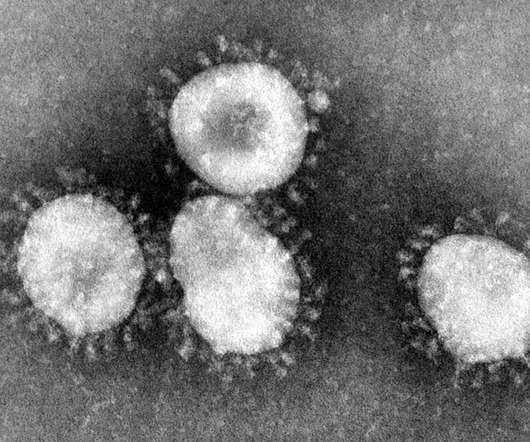
Researchers have developed a new vaccine technology that can provide protection against a broad range of coronaviruses with potential for future disease outbreaks – including ones we don’t know about yet. By training the immune system to attack these regions, it gives protection against other coronaviruses not represented in the vaccine.

































Let's personalize your content