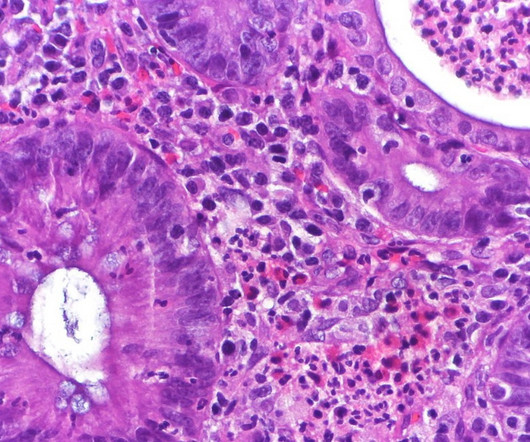
How Common Is Salmonella in Chicken? New USDA Regulations Target Frozen Products
XTalks
MAY 3, 2024
To combat food poisoning, US agriculture officials introduced a final rule last Friday, mandating significant reductions in Salmonella bacteria in specific chicken products. Starting in 2025, high levels of Salmonella in frozen breaded and stuffed chicken products will classify them as adulterated. How Common Is Salmonella in Chicken?

















































Let's personalize your content