Grand Rounds October 27, 2023: Digital, Decentralized and Democratized: Lessons From The Yale PaxLC Trial (Harlan M. Krumholz, MD, SM)
Rethinking Clinical Trials
OCTOBER 31, 2023
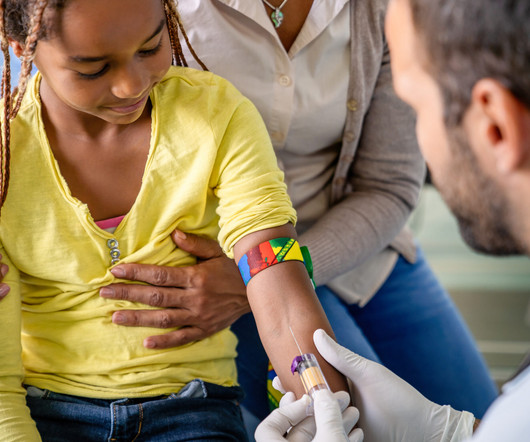
PaxLC brings together many innovations including online screening, digital medical record review, e-consent, home-delivery of medications, local clinical blood draws, home-based biospecimen collection, online diaries and surveys, digital medical record outcomes, and participant-centricity, and return of results. Pfizer provided feedback.















Let's personalize your content