Cannabinoids receptors: popular preclinical target but banned in 137 countries
Pharmaceutical Technology
DECEMBER 5, 2022
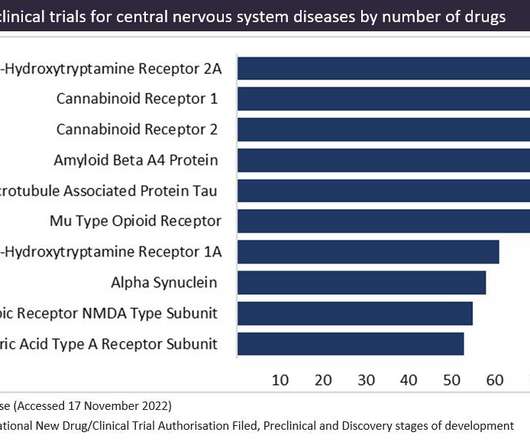
In 2022, a surge in the pipeline has led to cannabinoid receptors becoming the most popular target in preclinical development. Figure 1 shows the targets in preclinical trials for central nervous system diseases, as sourced from GlobalData’s Drugs Database. Cannabinoid-based drugs are derived from compounds found in the cannabis plant.





















Let's personalize your content