FDA approves Teva Pharmaceuticals’ AUSTEDO XR for TD and HD chorea
Pharmaceutical Technology
MAY 30, 2024
The US FDA approved Teva Pharmaceuticals’ AUSTEDO XR as a once-daily treatment option for tardive dyskinesia and Huntington’s disease chorea.

Pharmaceutical Technology
MAY 30, 2024
The US FDA approved Teva Pharmaceuticals’ AUSTEDO XR as a once-daily treatment option for tardive dyskinesia and Huntington’s disease chorea.

Bio Pharma Dive
MAY 30, 2024
The $73 million round for CinRx Pharma, which created a startup AstraZeneca bought last year, will help fund subsidiaries making drugs for obesity, irritable bowel syndrome and gastroparesis.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
MAY 24, 2024
But the head of the FDA’s CBER office didn’t tip where the agency stands on potentially broadening use of Sarepta’s Duchenne gene therapy Elevidys.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 29, 2024
Home Editorial Services Interview Q&A Chronicle Specials Pharma Mart ePharmail Archives Join Pharma | Login Home > TopNews you can get e-magazine links on WhatsApp.

Rethinking Clinical Trials
MAY 29, 2024
Dr. Shruti Gohil In this Friday’s PCT Grand Rounds, Shruti Gohil of the University of California, Irvine, will present “The INSPIRE Abdominal and Skin/Soft Tissue Infection Trials: Intelligent Stewardship Prompts to Improve Real-Time Empiric Antibiotic Selection for Patients.” The Grand Rounds session will be held on Friday, May 31, 2024, at 1:00 pm eastern.

Outsourcing Pharma
MAY 30, 2024
In an intriguing interview with Cybinâs CEO, Doug Drysdale, OSP senior editor, Liza Laws found out how second-generation psychedelics could be the biggest breakthrough in psychiatry in 40 years.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 29, 2024
A substance found in foods like pomegranates, strawberries, and walnuts restored the ability to detect and remove damaged cells in mice modeling Alzheimer’s disease, scientists report in a new paper. The same research team previously found a form of vitamin B3 called nicotinamide riboside (NR) helps remove damaged mitochondria from the brain.

Rethinking Clinical Trials
MAY 28, 2024
Drs. Kevin Weinfurt, Lesley Curtis, and Adrian Hernandez, co–principal investigators of the Coordinating Center Leaders of the NIH Pragmatic Trials Collaboratory met in Bethesda, Maryland, on May 9 and 10 for the program’s 2024 Annual Steering Committee Meeting—an opportunity to network and hold rich discussions on key issues related to pragmatic research.
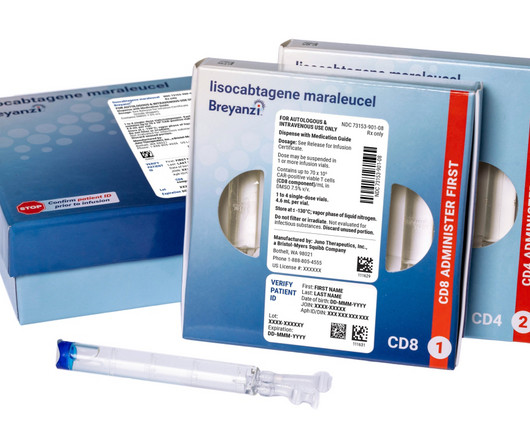
Fierce Pharma
MAY 30, 2024
In the years since gaining an initial FDA approval for Breyanzi, Bristol Myers Squibb has worked hard to expand the reach of its cell therapy. | In the years since gaining an initial FDA approval for Breyanzi, Bristol Myers Squibb has worked hard to expand the reach of its cell therapy. For the third time in as many months, those efforts have yielded a label expansion at the FDA.

Bio Pharma Dive
MAY 29, 2024
The Series B funding from Two Sigma Ventures, RA Capital and others will help Gameto develop technology it says could replace hormonal injections and shorten the IVF process.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
MAY 28, 2024
Scientists of QUT have secured A$4m ($2.65m) grant from the US DoD for developing new treatments for Parkinson's disease.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 27, 2024
The psychedelic drug psilocybin (as found in ‘magic’ mushrooms) has promising potential as a treatment for the eating disorder anorexia nervosa, according to new research that looked at its effects on an animal model of the condition.

Rethinking Clinical Trials
MAY 30, 2024
Complete materials are now available from the NIH Pragmatic Trials Collaboratory’s recent workshop, “Patient-Centered Research in Real-World Settings: Essentials of Embedded Pragmatic Clinical Trials.” The 1-day workshop, held on May 19 at the 45th annual Society for Clinical Trials meeting in Boston, introduced concepts in the design, conduct, and implementation of pragmatic clinical trials embedded in healthcare systems.
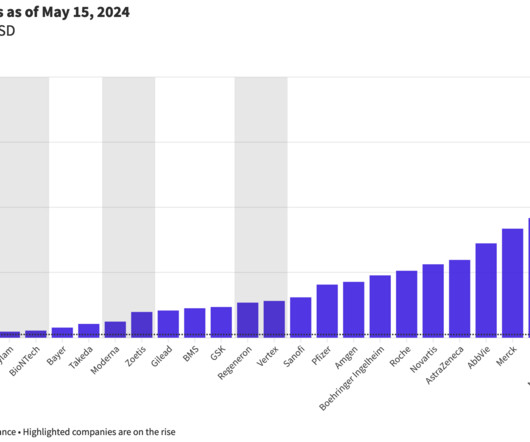
BioSpace
MAY 28, 2024
As AstraZeneca looks to climb toward the top of biopharma companies by revenue by the end of the decade, smaller companies are looking to join the ranks of the unofficial Big Pharma club.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Bio Pharma Dive
MAY 30, 2024
The company is prioritizing development of an earlier so-called FcRn inhibitor, a type of medicine that’s shown promise treating multiple inflammatory conditions.

Pharmaceutical Technology
MAY 24, 2024
The US FDA's EMDAC is set to hold a meeting to assess the benefit-risk profile of Novo Nordisk's insulin icodec, to treat type 1 diabetes.
AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 28, 2024
There are a lot of connected, moving biological parts in our brains, which makes the study of brain diseases particularly challenging. Now new research has shed light on a key brain process potentially involved in Alzheimer’s disease.

Fierce Pharma
MAY 28, 2024
A recently announced $900 million restructuring drive is already making waves at Takeda, wi | From early July to March 2025, the Japanese drugmaker will cut 495 staffers at its Cambridge location and 146 in Lexington, Massachusetts. Takeda is the largest life sciences employer in the state.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
pharmaphorum
MAY 30, 2024
COVID-19 vaccination rates have fallen precipitously in Europe and should be ramped up this autumn so they are at least in line with influenza vaccine uptake.

Bio Pharma Dive
MAY 28, 2024
The deal involves royalties for a brain cancer drug Agios previously sold off and that Royalty Pharma thinks could generate more than $1 billion in U.S. sales.

Pharmaceutical Technology
MAY 29, 2024
Prothena plans to initiate a Phase I trial in an undisclosed indication by the end of 2024.

BioSpace
MAY 29, 2024
As BioNTech struggles to establish its footing in a post-pandemic world, the biotech has secured $145 million from the Coalition for Epidemic Preparedness Innovations to expand its mRNA operations in Rwanda.

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.

Fierce Pharma
MAY 30, 2024
The United States Department of Health and Human Services has expanded its avian flu pandemic preparedness partnership with CSL Seqirus, lining up the vaccine specialist to

Antidote
MAY 30, 2024
One may choose to participate in a clinical trial for many reasons. Volunteering often has multiple benefits, from advancing medical research to gaining access to cutting-edge medical care. However, deciding to enroll in a clinical trial is only one part of the process.

Bio Pharma Dive
MAY 29, 2024
An acquisition of EyeBio will hand Merck a treatment for diabetic macular edema and age-related macular degeneration that’s ready for pivotal testing.

Pharmaceutical Technology
MAY 30, 2024
BioNTech and CEPI have announced an expansion of their strategic partnership, aiming to strengthen the mRNA vaccine ecosystem in Africa.

BioSpace
MAY 27, 2024
Approaches and targets for depression and other mental health illnesses have remained stagnant for decades. With several readouts for novel therapies on the horizon, that could be changing.

pharmaphorum
MAY 27, 2024
The overall survival (OS) data has come in from the TROPION-Lung01 study of AstraZeneca and Daiichi Sankyo’s datopotamab deruxtecan (Dato-DXd) in lung cancer – and the result likely isn’t what they were hoping for.

XTalks
MAY 28, 2024
The traditional clinical trials model is facing scrutiny. Marked by high costs ranging from $1 billion to $2 billion, lengthy timelines extending five to ten years and success rates falling below ten percent, there is a pressing need for reform. These daunting figures highlight the necessity for innovative solutions that can accelerate the journey of new drugs from the lab to patients.

Bio Pharma Dive
MAY 30, 2024
With “no playbook out there” to fall back on, the new company is taking a long-term approach as it develops its portfolio, an Otsuka Precision Health exec told MedTech Dive.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Let's personalize your content