Pfizer, with new study results, seeks to bring RSV shot to adults aged 18-59
Bio Pharma Dive
APRIL 9, 2024
The company plans to submit the trial data to regulators in a bid to win approval of its vaccine Abrysvo in adults as young as 18 years old.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 adults
adults 
Bio Pharma Dive
APRIL 9, 2024
The company plans to submit the trial data to regulators in a bid to win approval of its vaccine Abrysvo in adults as young as 18 years old.

Pharmaceutical Technology
JANUARY 30, 2024
The EMA has accepted GSK’s regulatory application seeking expansion of its RSVvaccine, Arexvy, to include adults aged 50 to 59 years.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 31, 2022
After decades of setbacks, the respiratory syncytial virus (RSV) vaccine field has bounced back with positive Phase III trial results in older adults. GSK’s Phase III pivotal trial included roughly 25,000 adults aged 60 years and older across 17 countries. in adults aged 70-79 years and 94.6%
Pharmaceutical Technology
JULY 25, 2023
Boehringer Ingelheim and Eli Lilly have secured approval from the EC for Jardiance to treat adults with chronic kidney disease (CKD).

JAMA Internal Medicine
MAY 5, 2024
This randomized clinical trial seeks to determine the efficacy and safety of cytisinicline vs placebo to produce abstinence from e-cigarette use in adults seeking to quit vaping nicotine.
Bio Pharma Dive
OCTOBER 15, 2021
Agency advisers unanimously supported offering a second dose of J&J's coronavirus vaccine to adults who previously received it, though guidance could change as regulators evaluate mixing boosters.
Bio Pharma Dive
SEPTEMBER 17, 2021
In a back-and-forth meeting Friday, the committee opposed clearing a third shot widely for use in people older than 16, but agreed the benefits outweighed the risks for adults over 65 or those at risk of severe COVID-19.

Pharmaceutical Technology
FEBRUARY 7, 2024
The US FDA has accepted for review an sBLA to expand the usage of GSK’s RSV vaccine, Arexvy, in adults aged 50-59 years.

Pharmaceutical Technology
JUNE 24, 2022
This data comprises two Phase III clinical trials: PREVENT-19, which enrolled nearly 30,000 adult subjects in the US and Mexico as well as a UK-based trial with 15,000 adults. The post Novavax’s Covid-19 vaccine gets Taiwan FDA EUA for use in adults appeared first on Pharmaceutical Technology.
Pharmaceutical Technology
JUNE 6, 2023
American Regent and Daiichi Sankyo have announced that the INJECTAFER (ferric carboxymaltose injection) has received approval from the US Food and Drug Administration (FDA) to treat iron deficiency in adult patients with heart failure. It is also indicated for IDA adult patients with non-dialysis-dependent chronic kidney disease.
Bio Pharma Dive
NOVEMBER 9, 2021
Agency advisers had opposed a broad booster dose clearance in September, leading the FDA to limit use to older adults and those at high risk of COVID-19.

JAMA Internal Medicine
APRIL 14, 2024
This cross-sectional study examines the frequency and severity of acute cardiac events in hospitalized adults aged 50 years or older with respiratory syncytial virus infection.
Pharmaceutical Technology
OCTOBER 26, 2023
GSK plans to submit the data to regulatory agencies to support label expansion for Arexvy in the younger adult patient population in 2024.

Pharmaceutical Technology
JUNE 29, 2022
The Japanese Ministry of Health, Labour and Welfare (MHLW) has accepted GlaxoSmithKline’s (GSK) regulatory application for recombinant, adjuvanted Zoster vaccine, Shingrix, for preventing shingles (herpes zoster) in at-risk adults of the age 18 years and above.

Pharmaceutical Technology
AUGUST 25, 2023
The EC has granted marketing authorisation for Pfizer’s bivalent RSV prefusion F vaccine, Abrysvo, for pregnant women and older adults.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 28, 2023
India’s success story in pediatric vaccination programme should be further replicated in the form of adult vaccination programme, experts recommended on the occasion of the 2nd edition of the India Vaccine Leaders Conclave (IVLC) held between August 22 and August 23, 2023 under the theme “Building Resilient Vaccine Ecosystems” in Mumbai. (..)
Bio Pharma Dive
MARCH 29, 2022
Citing "some waning of protection over time," the agency authorized another dose of either Pfizer's or Moderna's shots for adults aged 50 years or older as well as for people with weakened immune systems.

JAMA Internal Medicine
JANUARY 7, 2024
This cohort study assesses the prevalence, underlying causes, and harms of diagnostic errors among hospitalized adults who died or were transferred to intensive care.

Medical Xpress
MAY 17, 2023
Scientists say tonsil removal is both cost- and clinically effective for adults who get recurrent severe sore throats.

JAMA Internal Medicine
MAY 29, 2023
This cohort study examines the association of intensive treatment of elevated blood pressure in hospitalized older adults with in-hospital clinical outcomes.

NPR Health - Shots
MARCH 6, 2023
Young Black adults, Mexican Americans and other Hispanic adults experienced the greatest cardiovascular risk factors, according to a study published in the Journal of the American Medical Association. Image credit: M. Spencer Green/AP)
Medical Xpress
MAY 3, 2023
These experiences, especially if they occur multiple times, have a devastating effect on the adult's emotional functioning, increasing the risk of depression, anxiety, addiction, or suicidality.

BioSpace
AUGUST 7, 2023
Data from a Phase III study released Tuesday found that Novo Nordisk’s Wegovy lowered the risk of cardiovascular complications and death by 20% versus placebo in overweight and obese adults.

Medical Xpress
APRIL 27, 2023
cigarette smoking dropped to another all-time low last year, with 1 in 9 adults saying they were current smokers, according to government survey data released Thursday. Meanwhile, electronic cigarette use rose, to about 1 in 17 adults.

pharmaphorum
OCTOBER 21, 2022
The UK company reveals the results from a partner-led survey to discover the rates of vaccination for adults, why levels are lower than expected, and what can be done to improve this. Why adult immunisation is important. Why adult immunisation is important. Addressing the issue. Vaccines central to GSK’s portfolio.
Medical Xpress
MAY 10, 2023
For adults with prurigo nodularis (PN), dupilumab, a fully human monoclonal antibody that blocks the shared receptor component for interleukin (IL)-4 and IL-13, demonstrates clinically meaningful and significant improvements in itch and skin lesions in two phase 3 trials (LIBERTY-PN PRIME and PRIME2), according to a study published online May 4 in (..)
Scienmag
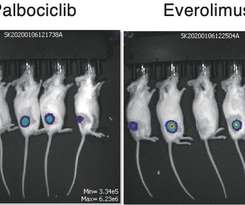
MARCH 31, 2022
Gene editing technology has been used to pinpoint new molecular targets for treating an aggressive form of leukemia in adults. November 24, 2021 Gene editing technology has been used to pinpoint new molecular targets for treating an aggressive form of leukemia in adults. Credit: Takashi Ishio, et al.

Medical Xpress
MARCH 6, 2023
New findings from researchers at Wake Forest University School of Medicine confirm that the rates of Type 1 and Type 2 diabetes continue to increase in children and young adults. Non-Hispanic Black and Hispanic children and young adults also had higher incidence rates of diabetes.

pharmaphorum
OCTOBER 21, 2022
Young adults with depression have been treated effectively with a cognitive behavioural therapy (CBT) course – delivered via text message – in a pilot study conducted by researchers in the US. NICE now also backs the use of digital CBT for adults with mild to moderate depression.

Scienmag
NOVEMBER 25, 2021
Pandemic depression persists among older adults: Study Credit: McMaster University Pandemic depression persists among older adults: Study Hamilton, ON (Nov.

NPR Health - Shots
AUGUST 24, 2022
The National Institutes of Health annually surveys substance abuse among young adults in its Monitoring the Future study. Image credit: Mary Altaffer/AP)
Medical Xpress
JANUARY 25, 2023
A healthy lifestyle, in particular a healthy diet, is associated with slower memory decline, finds a decade-long study of older adults in China, published today in The BMJ.
Medical Xpress
MARCH 28, 2023
Adults with atopic dermatitis (AD) have an increased risk for developing melanoma, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC), with significantly higher risks seen for moderate-to-severe versus mild AD, according to a study presented at the annual meeting of the American Academy of Dermatology, held from March 17 to 21 in New Orleans. (..)

Trialfacts
APRIL 25, 2024
This study aims to delve into the cellular mechanisms within fat tissue to enhance our understanding of how aging impacts the health of adults over time. Participants will be contributing valuable information that may benefit adults who are struggling with their health in the future.

Trialfacts
APRIL 25, 2024
This study aims to delve into the cellular mechanisms within fat tissue to enhance our understanding of how aging impacts the health of adults over time. Participants will be contributing valuable information that may benefit adults who are struggling with their health in the future.

Medical Xpress
FEBRUARY 7, 2023
The short-term effects of vaporized cannabis do not differ between adolescents and adults, while cannabidiol (CBD) does not dampen the effects of the drug, finds a new study led by UCL and King's College London researchers.
Medical Xpress
MAY 8, 2023
health policymakers to adopt routine testing of adults ages 40 and under for three genetic conditions posing high risk of life-threatening illness. An exhaustive cost-benefit analysis of population genetic testing published in Annals of Internal Medicine concludes with a recommendation to U.S.

JAMA Internal Medicine
FEBRUARY 25, 2024
This JAMA Network Insights reassesses the approach to caring for older adults with diabetes in the context of newly available pharmacologic agents.

JAMA Internal Medicine
OCTOBER 22, 2023
This cohort study assesses whether endogenous and exogenous thyrotoxicosis is associated with higher risk of cognitive disorders in adults 65 years and older.

Medical Xpress
MAY 1, 2023
Young adults who beat cancer face unique challenges later on in their adult lives. These include both psychological and physical impacts, such as body image disruption, social relationship difficulty, fertility and sexual distress, anxiety, depression and fear of cancer recurrence.

Pharma Times
AUGUST 26, 2022
It is estimated that RSV infections in older adults account for 177,000 hospitalisations and 14,000 deaths each year in the US alone

Medical Xpress
JANUARY 20, 2023
The national uninsurance rate for adults under age 65 with schizophrenia decreased by 50% after the implementation of the Affordable Care Act (ACA) in 2014, according to University of Massachusetts Amherst research published this week in JAMA Psychiatry.

Medical Xpress
MARCH 28, 2023
The World Health Organization said Tuesday it is no longer recommending additional COVID-19 vaccine booster doses for regular, medium-risk adults, saying the benefit was marginal.
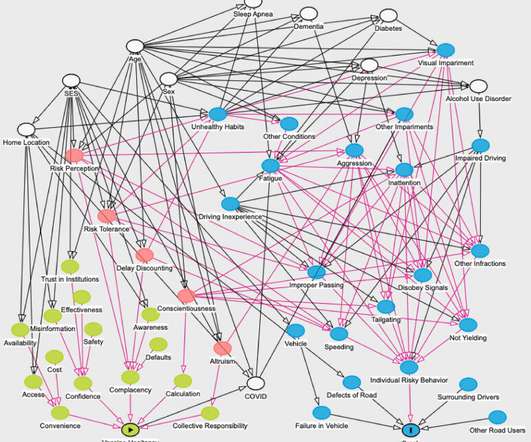
Medical Xpress
DECEMBER 12, 2022
Researchers found that adults who neglect these health recommendations may also neglect basic road safety. Reasons underlying hesitancy to get vaccinated against COVID-19 may be associated with increased risks of traffic accidents according to a new study in The American Journal of Medicine.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content