HIV vaccine being developed by Johnson & Johnson fails clinical trial
STAT News
JANUARY 18, 2023
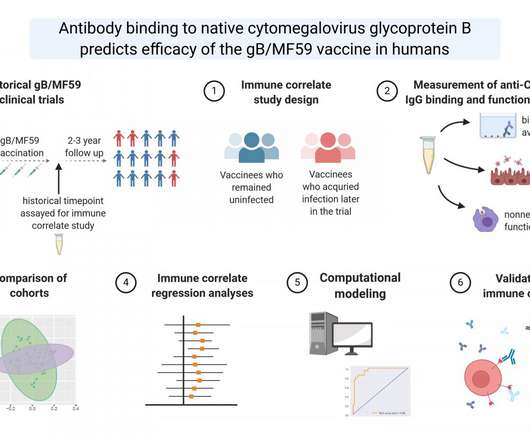
Yet another experimental HIV vaccine has failed. The National Institute of Allergy and Infectious Diseases reported Wednesday that a Phase 3 clinical trial of a vaccine was stopped because the vaccine was ineffective at preventing HIV infection. Read the rest…































Let's personalize your content