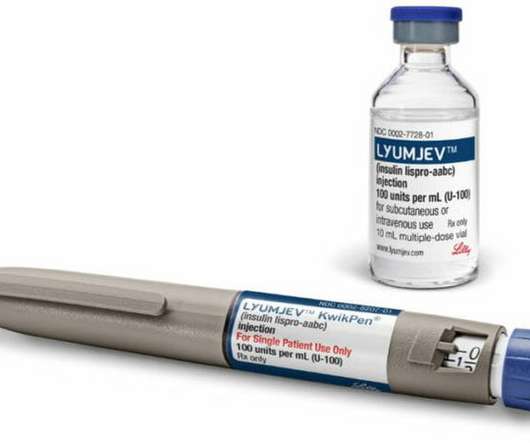
FDA approves Lyumjev® (insulin lispro-aabc injection) 100 units/mL for use in insulin pumps
The Pharma Data
AUGUST 17, 2021
approval of pump use for Lilly’s novel insulin is latest development designed to help people with diabetes manage blood sugar levels. Lyumjev, a novel formulation of insulin lispro developed to speed the absorption of insulin into the bloodstream and reduce A1C levels, was approved by the FDA in June 2020.











Let's personalize your content