RVAC and University of Pennsylvania to develop mRNA vaccines
Pharmaceutical Technology
MARCH 20, 2023
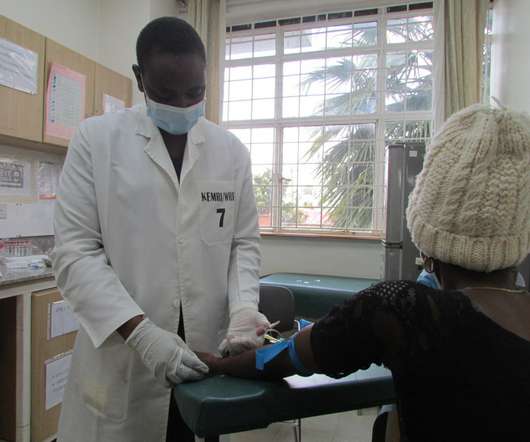
RVAC Medicines has announced a research collaboration with the University of Pennsylvania (Penn) for the discovery and development of mRNA vaccines. The mRNA vaccine candidates will help reduce the chances of autoimmune responses that might lead to allergic conditions or serious autoimmune diseases.



































Let's personalize your content