UK MHRA grants authorisation for Moderna’s Covid-19 booster vaccine
Pharmaceutical Technology
AUGUST 15, 2022
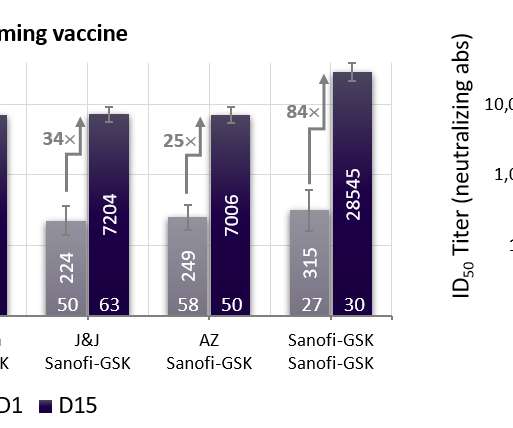
The updated, Omicron-containing bivalent vaccine that acts on two coronavirus variants is indicated as a booster dose for active immunisation for the prevention of Covid-19 in people of this age group. The latest MHRA decision is based on findings from the Phase II/III clinical trial, where the mRNA-1273.214 vaccine met all primary endpoints.








































Let's personalize your content