Biopharmaceutical Contract Manufacturing: Transforming Promise into Reality
Roots Analysis
AUGUST 29, 2023
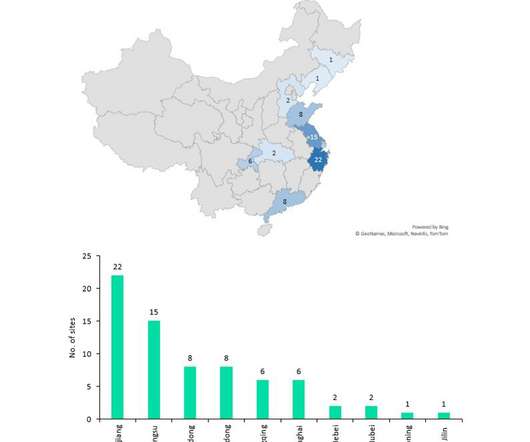
In this context, it is worth highlighting those 14 biopharmaceutical products ( including cell therapies, gene therapies, monoclonal antibodies and recombinant proteins ) were approved in the US alone, in 2021. As a result, biopharmaceutical contract manufacturing has picked up pace in the recent years.























Let's personalize your content