US launches $5bn effort for new Covid-19 vaccines development
Pharmaceutical Technology
APRIL 11, 2023
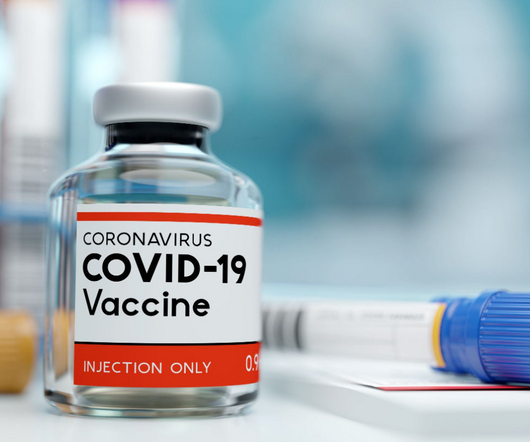
The US government is reportedly investing more than $5bn in a programme to accelerate the development of new Covid-19 vaccines and treatments. Health experts have lent their support to the new programme as the existing vaccines may not be as effective against future virus threats.



























Let's personalize your content