Latest Data from Novavax COVID-19 Vaccine Trial Involving B.1.351 Variant Shared by Protein Vaccine Maker
XTalks
MAY 7, 2021
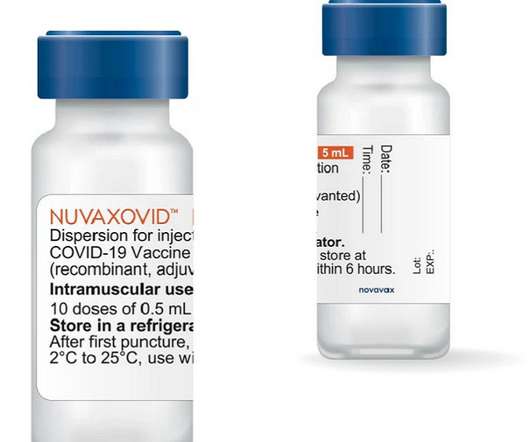
As Novavax awaits emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for its protein-based COVID-19 vaccine NVX-CoV2373, the company shared new data this week on the vaccine’s efficacy against the South African B.1.351 1.351 variant.









































Let's personalize your content