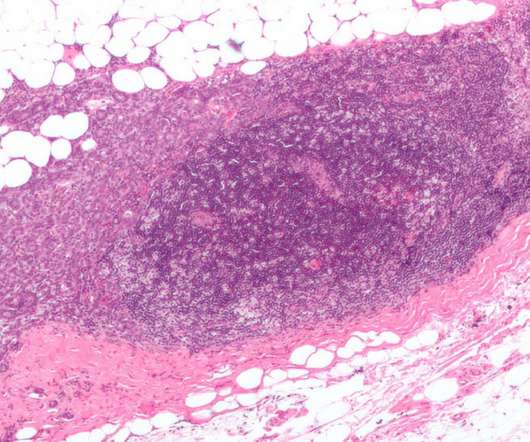
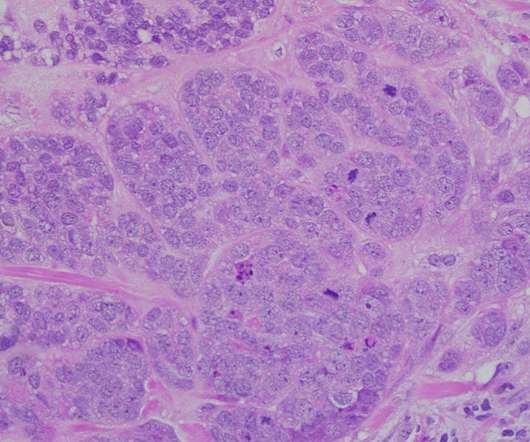
SABCS: Pharmas show their working in HR+/HER2- breast cancer
pharmaphorum
DECEMBER 12, 2022
The San Antonio Breast Cancer Symposium (SABCS) has highlighted some of the most promising clinical research being undertaken by drugmakers in the category, with AstraZeneca leading the charge with a trio of major trial readouts. The post SABCS: Pharmas show their working in HR+/HER2- breast cancer appeared first on.









































Let's personalize your content