IQVIA’s report card for clinical trial diversity: must do better
pharmaphorum
NOVEMBER 11, 2022
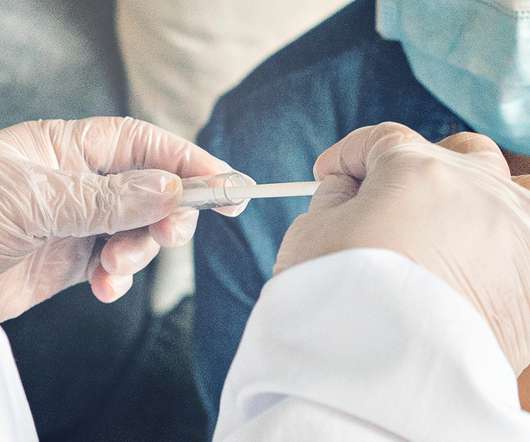
The lack of diversity in clinical trials has been a topic of debate for decades, but was thrust into the spotlight as the impact of the pandemic on poorer, less educated and ethnically diverse populations became even more apparent. The post IQVIA’s report card for clinical trial diversity: must do better appeared first on.
























Let's personalize your content