Digital tools driving innovative clinical trials
pharmaphorum
JULY 8, 2022
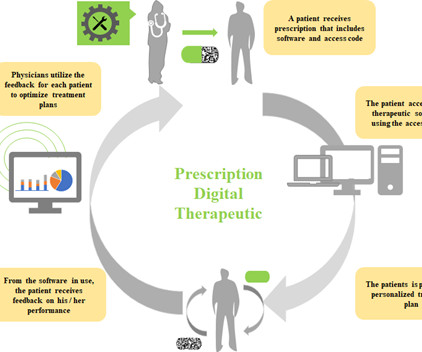
The COVID-19 pandemic has catalysed significant changes in the way pharma develops drugs, particularly in the clinical trial space. Hybrid or decentralised clinical trials (DCTs) have gained traction as technology, infrastructure and knowledge have evolved to support their use. Source: Izmailova et al, 2017.












































Let's personalize your content