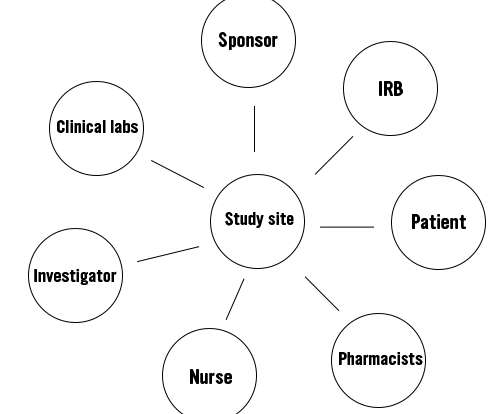
Changing Perspectives on Clinical Research Through Mindfulness and Professionalism
ACRP blog
MAY 6, 2024
Besides her complicated duties as a doctor in neurology at the time, she came to realize that esophageal precancerous lesions were wearing on her body, leading to even greater stressful thoughts about how the health crisis would affect her career and what others would think of her, and eventually to burnout.











































Let's personalize your content