Phesi’s AI-driven Trial Accelerator platform contains over 100 million patients
Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development

Rethinking Clinical Trials
OCTOBER 2, 2023
The Patient-Centered Outcomes Core has developed a new tool kit to provide resources to support the capture of patient-reported outcome (PRO) measures in diverse study populations. The post October 2, 2023: Patient-Centered Outcomes Core Develops Tool Kit to Promote Health Equity in PROs appeared first on Rethinking Clinical Trials.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
JANUARY 14, 2022
The European Commission, EMA and national regulators within the EU have launched an initiative to change the way clinical trials are designed and run in order to position the bloc as an international “focal point” for clinical research.

XTalks
MAY 4, 2023
When a person with celiac disease eats something that contains gluten, their immune system attacks their small intestine, damaging the lining and interfering with the absorption of nutrients from food. However, there are ongoing clinical trials for celiac disease to investigate potential new treatments.
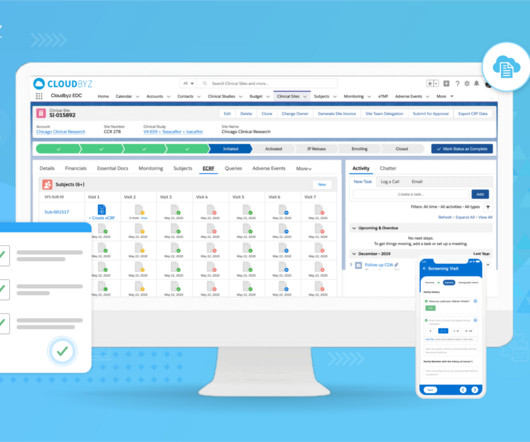
Cloudbyz
JUNE 16, 2023
Clinical trials are crucial for advancing medical research and developing innovative treatments. Effective clinical trial data archiving is essential to ensure data integrity, regulatory compliance, and seamless access. Effectively managing and storing such large datasets presents logistical challenges.
Cloudbyz
SEPTEMBER 16, 2023
Addressing data privacy and data protection concerns when implementing ChatGPT in clinical trial operations management is crucial to maintain compliance with regulations, safeguard sensitive patient information, and build trust among stakeholders.

Drug Discovery World
AUGUST 4, 2022
It may seem counterintuitive to spend time and money on planning for containment and delivery systems for a drug in the earliest stages of discovery when the chances of that molecule making it to market are still quite low. However, starting early can greatly improve the efficiency and speed of the development process. Never too early.

CTTI (Clinical Trials Transformation Initiative)
JANUARY 10, 2024
Timely, accurate, and complete registration and reporting of summary results information for applicable clinical trials on ClinicalTrials.gov allows access to current research and evidence for all partners in the clinical trials enterprise, including patients, providers, sponsors and investigators, regulators, payers, and health system leaders.

Cloudbyz
APRIL 9, 2023
Clinical trial management involves various activities and processes that can have a significant impact on the environment and contribute to sustainability issues. Clinical trials, in particular, have a significant impact on the environment, and it is essential to address this impact to ensure a sustainable future.
pharmaphorum
AUGUST 6, 2021
Patient enrollment is a common issue across many therapeutic areas in clinical research. Psychiatric disorders in particular represent a trial area with significantly high drop-out rates and poor patient recruitment. Psychiatric disorders have historically had great difficulty onboarding and retaining patients for clinical trials.
Drug Discovery World
AUGUST 9, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted Aleta Biotherapeutics a clinical trial authorisation (CTA) to evaluate biologic ALETA-001 in a Phase I/II clinical trial in patients with B-cell malignancies who are relapsed/refractory to CD19 CAR T cell therapy.
Pharmaceutical Technology
OCTOBER 6, 2022
from the US National Institutes of Health (NIH) unit National Institute of Allergy and Infectious Diseases (NIAID) to develop a prophylactic intranasal vaccine against Neisseria gonorrhoeae (NG). Leveraging its outer membrane vesicles (OMV) platform technology, Intravacc will develop the vaccine.
Pharmaceutical Technology
DECEMBER 12, 2022
Gilead company Kite has entered an international strategic partnership with Arcellx for the joint development and commercialisation of the latter’s T-cell therapy, CART-ddBCMA, to treat relapsed or refractory multiple myeloma patients. Additionally, Kite will make other potential payments to Arcellx.

Pfizer
FEBRUARY 16, 2023
These study participants, representing approximately half of the total recruited participants in the trial, are being discontinued following violations of Good Clinical Practice (GCP) at certain clinical trial sites run by a third-party clinical trial site operator.

Worldwide Clinical Trials
FEBRUARY 21, 2024
During clinical development, new chemical entities (NCEs) require an absorption, metabolism, and excretion (AME) study. Regardless of the formulation, the entire dose must be administered to each subject, and the dosing containers must be checked for residual radioactivity.

XTalks
NOVEMBER 7, 2022
Gleich, MD, FACS, Senior Vice President, Medical Department, and Dr. Christopher Huth, PhD, Senior Clinical Trial Manager, Clinical Trial Management. Liquid Biopsy Use in Oncology Clinical Trials. How personalized, targeted assays can be developed to detect somatic mutations from cfDNA. “A
XTalks
APRIL 25, 2024
Now we recognize that over the last ten years or so there have been major developments in pharmacotherapy for the management of different etiologies of heart failure,” says Dr. Nicholas Alp, MD, PhD, FACC, FRCP, Vice President of the Medical Department at Medpace. Survival rates were also impressive, exceeding 90 percent at one year.

Drug Discovery World
FEBRUARY 1, 2023
But more robust studies are needed across the board to help with the discovery and development of cannabis-based pharmaceuticals. Big pharma is helping to shape the focus The good news is that clinical trials are on the increase and market education is upping pace.

XTalks
NOVEMBER 6, 2020
Canadian clinical-stage biotech company Symvivo Corporation has developed an oral COVID-19 vaccine that entered clinical trials this week. The first healthy volunteer was dosed with the vaccine in Australia as part of the bacTRL-Spike COVID-19 Phase I clinical trial. COVID-19 Clinical Trials.

XTalks
NOVEMBER 29, 2022
Xtalks interviewed Devon Adams to learn more about his work related to decentralized oncology trials as a senior analyst for legislative policy specific to clinical trials at the ACS CAN. Read on to learn more! Over 1,100 cancer patients and cancer survivors responded.
Advarra
FEBRUARY 24, 2023
In most cases, the results you are really looking for and trying to prove in a trial can be complex. One area receiving increased focus from the Food and Drug Administration (FDA) are trial designs incorporating multiple endpoints to support efficacy. All key questions to consider and address before the therapy even leaves the lab.

Pharmaceutical Technology
SEPTEMBER 20, 2022
Unlike commercial pharmaceutical packaging, the primary consideration in clinical trial packaging is protecting the product quality and reliability for research. Finding the best clinical trial packaging services providers. Clinical trial packaging and labelling solutions.
Drug Discovery World
OCTOBER 31, 2023
UK regulatory authorities have approved the first trial of a gene therapy for young children with Hunter syndrome. The drug was developed over eight years by Brian Bigger, Professor of Cell and Gene Therapy at The University of Manchester.

The Pharma Data
JANUARY 13, 2021
. Psychedelic Pharmaceutical Scientist and Clinical Pharmacologist Robert Barrow Appointed as Chief Development Officer. Mr. Barrow has over a decade of experience leading drug development programs aimed at identifying and testing novel treatments in a wide range of disease conditions under FDA and EMA.

The Pharma Data
MARCH 31, 2022
A Phase 2 clinical trial evaluating various additional COVID-19 booster shots has begun enrolling adult participants in the United States. “This trial will help us understand if we can use prototype and variant vaccines alone or together to shift immune responses to cover existing and emerging COVID-19 variants.”

XTalks
APRIL 24, 2023
Melanie Blank, clinical team leader for General Medicine Branch 1 at the US Food and Drug Administration’s (FDA) Division of Clinical Evaluation and Pharmacology/Toxicology (DCEPT), the agency is seeing one or two new applications coming in every week for new gene therapies for different diseases.

STAT News
SEPTEMBER 26, 2022
Here’s a scenario anyone who has done clinical research will recognize: A 32-year-old woman participating in a Phase 1 healthy-volunteer crossover clinical trial tested negative for pregnancy when she enrolled and agreed to use contraception during the course of the trial, as specified in the protocol.

Worldwide Clinical Trials
SEPTEMBER 29, 2022
Ensuring a seamless transition from preclinical to clinical stages in large molecule bioanalysis will help you reach crucial trial milestones on time and within budget. However, optimizing pharmacokinetics (PK) assays to bridge the preclinical-to-clinical gap requires some finesse. anti-human IgG).
The Pharma Data
APRIL 3, 2021
The CoVIg-19 Plasma Alliance today announced that the Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), did not meet its endpoints. About the ITAC Trial.

pharmaphorum
DECEMBER 4, 2022
Clinical trials of a wireless brain chip developed by Elon Muck’s Neuralink company will be tested in human volunteers within the next six months – and Musk himself says he intends to have one implanted for a future demo event. The post Elon Musk’s Neuralink brain interface chip set for human trials appeared first on.

The Pharma Data
JANUARY 10, 2021
Each of the first three dose cohorts contain up to six patients on ASLAN004 and two patients on placebo, and the expansion cohort will contain at least 12 patients on ASLAN004 and at least six patients on placebo. This release contains forward-looking statements. SINGAPORE, Jan. Forward looking statements.

The Pharma Data
JANUARY 3, 2021
. United Kingdom Medicines and Healthcare Products Regulatory Agency authorized Clinical Trial Application. With these important regulatory clearances for our first-in-human clinical trial for INZ-701 in subjects with ENPP1 deficiency, we have transitioned from a research-stage to a clinical-stage company.

The Pharma Data
OCTOBER 19, 2020
NASDAQ: CNSP) (“CNS” or the “Company”), a biopharmaceutical company specializing in the development of novel treatments for primary and metastatic cancers of the brain and central nervous system, today announced that the Company’s U.S. Phase 2 trial for Berubicin during the first quarter of 2021.”
The Pharma Data
JULY 8, 2021
The update includes an addition to the Indications and Usage section of the label (Section 1) to emphasize the disease stages studied in the clinical trials, as seen below ( italics to note updated language). Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trial(s).

Worldwide Clinical Trials
JUNE 15, 2022
At the end of May, we hosted a webinar titled “ Changing Times, Changing Therapies: Keeping Up with Advancements in Cell and Gene Therapies ” to provide a quick update on the latest advancements and ongoing in development of these advanced therapeutics. Clinical holds are becoming more common, especially in gene therapy programs.
Drug Discovery World
OCTOBER 9, 2023
Positive Phase I trial data indicates that Reqorsa (quaratusugene ozeplasmid) has shown early efficacy for the treatment of non-small cell lung cancer (NSCLC). Developed by gene therapy company Genprex, Reqorsa is a non-viral gene therapy that leads to expression of the TUSC2 tumour suppressor gene in cancers.

Drug Discovery World
DECEMBER 6, 2022
As a result of clear US Food and Drug Administration (FDA) guidelines, the leading vaccine development programmes for Covid-19 were all remarkably similar. Indeed, few randomised trials of NPIs have been undertaken. Quasi-experimental and other randomised trial designs . Pre-specification of effect size .

The Pharma Data
JANUARY 17, 2021
The treatment is based on the Grifols immunoglobulin Gamunex®-C, and contains anti-SARS-COV-2 polyclonal antibodies from plasma donors who have recovered from COVID-19. It could also help contain outbreaks in places where the vaccination hasn’t begun or is still underway. BARCELONA, Spain , Jan. and on the U.S.

Pharmaceutical Technology
SEPTEMBER 14, 2022
Pharmaceutical drug research and development (R&D) activities are capital-intensive, which makes the outsourcing of clinical dose manufacturing and marketing popular. Find the leading clinical dose companies in contract marketing. Prospects of clinical dose companies in contract marketing.

Drug Discovery World
MARCH 28, 2023
SolasCure’s first investigational product, Aurase Wound Gel, a hydrogel containing an enzyme cloned from medical maggots which aims to accelerate wound debridement, is due to enter further Phase II efficacy-supporting trials. funding to develop Aurase Wound Gel appeared first on Drug Discovery World (DDW).

The Pharma Data
JANUARY 25, 2021
agree to place handheld Ceribell Rapid Response EEG systems in hospitals in connection with its Phase 3 RSE clinical trial. Within the RAISE trial, it will allow clinicians to rapidly establish a diagnosis of nonconvulsive SE in the absence of immediate availability of conventional EEG. About the RAISE Trial. RADNOR, Pa.–(

pharmaphorum
JANUARY 27, 2023
Late last year, pharmaphorum caught up with Dr Karen Mullen, chief medical officer and VP of clinical & medical affairs at global drug development consultancy Boyds. Clinical trials design and patient input The definition of patient centricity, in fact, and its benefits are now – finally – being defined by patients themselves. “We
pharmaphorum
FEBRUARY 24, 2022
Clinical stage pharmaceutical company Cantex Pharmaceuticals has obtained a global licence from Harvard University’s Office of Technology Development to develop the small-molecule drug azeliragon into a treatment for inflammatory lung diseases, including COVID-19. said Cantex CEO Stephen Marcus.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Still, navigating such patent issues during drug development can be difficult, and Camargo can assist in finding a safe course. Ken Phelps.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content